0774
In-vivo application of a trained neural network using a fusion of computational fluid dynamic and 4D flow MRI data1Radiology, University of Wisconsin, Madison, WI, United States, 2Mechanical Engineering, University of Wisconsin, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin, Madison, WI, United States, 4Medical Physics, University of Wisconsin, Madison, WI, United States
Synopsis
Augmentation of 4D flow MRI data with computational fluid dynamics (CFD) -informed training networks may provide a method to produce highly accurate physiological flow fields. In this preliminary work, the potential utility of such a method was demonstrated by using high resolution patient-specific CFD data to train a neural network, and then using the trained network to enhance MRI-derived velocity fields.
INTRODUCTION
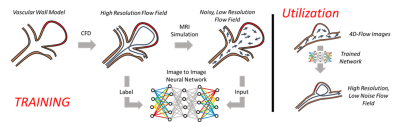
4D flow MRI-derived metrics have potential for improving cardiovascular disease diagnosis and treatment planning(1,2); however, patient scan time restrictions limit MRI flow resolution, and inherent flow encoding properties lead to imperfections in the flow field. Utilization of computational fluid dynamics (CFD) may address some of these limitations, providing high resolution, low noise velocity fields which satisfy the physics of fluid flow (mass and momentum conservation). Yet, standalone CFD can also be limited due to its dependence on patient-specific input conditions, and errors which propagate from inaccurate assumptions. A method that utilizes the best of both MRI and CFD may allow for enhanced flow analysis(3,4). Therefore, the purpose of this work was to develop a machine learning paradigm which fuses information from 4D flow MRI and CFD using supervised learning, to provide high resolution, physics-based, patient-specific flow fields.METHODS
An overview of the study methods is shown in Figure 1. To begin, a large data set was created for neural network training by performing 180 unique CFD simulations (Figure 2) on cerebrovascular geometries in CONVERGE CFD Software (Convergent Science, Madison, WI). These high-resolution flow fields were used in a GPU augmented MRI simulation which included synthetic background signal, random rotations and scaling, and Fourier sampling with complex gaussian noise. The modified flow fields were then used to train a convolutional neural network (CNN) with architecture similar to that used in advanced super resolution approaches(5-8). Training was blockwise using a 32 cubic input patch in the high resolution CFD domain and a mean square error loss metric was used (PyTorch, Open Source).The trained CNN was evaluated in two stages. The first stage involved comparison of trained flow data to high resolution 4D flow MRI phantom data. To do this, a cerebrovascular phantom was integrated into a flow loop with a pulsatile flow system (PD-1100, BDC Laboratory, Wheat Ridge, CO). The model was then scanned on a 3T MR imaging system (Signa Premier, GE Healthcare, Waukesha, WI) with PC-VIPR(9). Imaging parameters included a 0.41mm isotropic spatial resolution, 256mm field of view, 9ms repetition time, 100 cm/s velocity encoding, and 24 minute scan time. Data was subsampled into 6- and 12-minute acquisitions. The trained network was applied to sub-sampled data and the resulting flow field was compared to the 24-minute high resolution standard with a root mean square error (RMSE) metric.
The second evaluation stage involved testing the trained network on cerebral time-averaged 4D flow MRI data (acquired with PC-VIPR) from 10 patients. Vessel boundaries were manually segmented from each patient flow case, and flow metrics of both pre-and post-trained data sets were analyzed in Ensight (CEI, Apex, NC, United States). Near-wall velocity gradients were calculated at the vessel mask boundary by considering the change in velocity in a direction normal to the vessel wall, vorticity was calculated by taking the curl of velocity vectors throughout the vessel volume, and flow was recorded at a plane placed in the vessel cross-section.
RESULTS
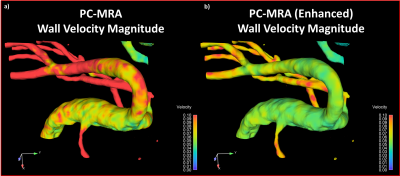
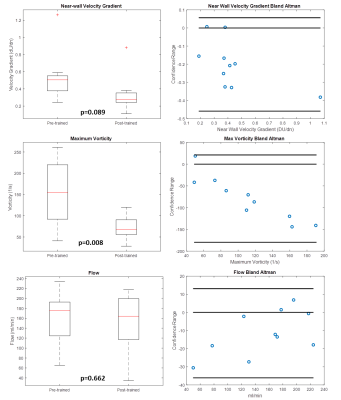
Application of the trained network to the experimental physical phantom data produced a significant reduction in background noise (Figure 3). When the high resolution 24-minute 4D flow MRI raw velocity was taken as the reference data set, RMSE was reduced from 2.55 to 1.49 in the post-training 12-minute sub-sample scan data, and from 4.06 to 1.64 in the post-training 6-minute sub-sample scan data.As with the experimental test case, CNN-enhanced in-vivo velocity images had lower noise and higher apparent spatial resolution than the raw velocity images. Additionally, the CNN-enhanced patient data had greater vessel boundary delineation. Accordingly, the velocity representation was also improved in CNN-cleaned images, particularly near the vessel walls (Figure 4). Application of the trained network in the ten patient data sets produced decreases in near-wall velocity gradient estimation (p=0.089) and vorticity estimation (p=0.008), but no difference in bulk flow estimation (p=0.662) when compared to pre-training data (Figure 5).
DISCUSSION
The fusion of CFD and 4D flow MRI data with machine learning successfully reduced image noise and decreased velocity-related errors in both phantom test cases and patient specific data sets. Near wall velocity gradients (the major component in wall shear stress calculations) were consistently lower in the post-trained patient data, and adhered more closely to the physical laws of fluid flow. Additionally, vorticity metrics were consistently lower in post-trained data. We presume that the bulk of vorticity reduction was a result of the lower noise levels in post-trained images. Furthermore, Bulk flow quantification was not sacrificed at the expense of improved small scale metric quantification. Application of this trained network is somewhat limited to cerebral flow data, as this is the type of data that was used to train the network. However, future work is planned to expand the training data and apply the trained network to a wider variety of biological flow regimes.CONCLUSION
In this preliminary work, a machine-learning-enabled CFD and PC-MRI hybrid method was demonstrated. While phantom experimentation provided comparison standards for this methodology that will be built upon in future work, results of network testing on human subject 4D flow MRI data demonstrated the potential utility of this proposed method in minimizing error in clinically relevant flow parameters.Acknowledgements
This work was supported by a ML4MI pilot grant from the UW Departments of Radiology and Medical Physics, and the Grainger Institute for Engineering. Support was also received from the National Institutes of Health NRSA T32 training grant (DR) from the NHLBI. The authors also wish to acknowledge support from GE Healthcare who provides research support to the University of Wisconsin.References
1. Sengupta PP, Pedrizzetti G, Kilner PJ, et al. Emerging trends in CV flow visualization. JACC Cardiovasc Imaging 2012;5(3):305-316.
2. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012;36(5):1015-1036.
3. Bakhshinejad A, Baghaie A, Vali A, Saloner D, Rayz VL, D'Souza RM. Merging computational fluid dynamics and 4D Flow MRI using proper orthogonal decomposition and ridge regression. J Biomech 2017;58:162-173.
4. Petersson S, Dyverfeldt P, Ebbers T. Assessment of the accuracy of MRI wall shear stress estimation using numerical simulations. J Magn Reson Imaging 2012;36(1):128-138.
5. HG C, XH H, C R, LB Q, QZ T. CISRDCNN: Super-resolution of compressed images using deep convolutional neural networks. Neurocomputing 2018;285:204-219.
6. YS L, J H, X Z, WY X, JJ L. Hyperspectral image super-resolution using deep convolutional neural network. Neurocomputing 2017;266:29-41.
7. K S, A A, A S. Super-Resolution of Magnetic Resonance Images using Deep Convolutional Neural Networks. Ieee Int C Electr Ta 2017.
8. XB S, YC D, XY Q. Deep Depth Super-Resolution: Learning Depth Super-Resolution Using Deep Convolutional Neural Network. Computer Vision - Accv 2016;10114:360-376.
9. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008;60(6):1329-1336.
Figures