0772
Deep Learning-based Strain Quantification from CINE Cardiac MRI
Teodora Chitiboi1, Bogdan Georgescu1, Jens Wetzl2, Indraneel Borgohain1, Christian Geppert2, Stefan K Piechnik3, Stefan Neubauer3, Steffen Petersen4, and Puneet Sharma1
1Siemens Healthineers, Princeton, NJ, United States, 2Magnetic Resonance, Siemens Healthcare, Erlangen, Germany, 3Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 4NIHR Biomedical Research Centre at Barts, Queen Mary University of London, London, United Kingdom
1Siemens Healthineers, Princeton, NJ, United States, 2Magnetic Resonance, Siemens Healthcare, Erlangen, Germany, 3Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 4NIHR Biomedical Research Centre at Barts, Queen Mary University of London, London, United Kingdom
Synopsis
Deep learning enables fully automatic strain analysis from CINE MRI on large subject cohorts. Deep learning neural nets were trained to segment the heart chambers from CINE MRI using manually annotated ground truth. After validation on more than 1700 different patient datasets, the models were used to generate segmentations as the first step of a fully automatic strain analysis pipeline for 460 subjects. We found significant differences associated with gender (strain magnitude smaller for males), height (lower strain magnitude for patients taller than 170 cm) and age (lower circumferential and longitudinal strain for subjects older than 60 years).
Introduction
MRI enables accurate quantification of cardiac chamber volumes and function. Fully automatic cardiac function assessment from CINE MRI has yet to become a standard in clinical practice, but deep learning approaches show increasing potential when leveraging great amounts of annotated data. We trained deep neural networks to perform joint left and right ventricle segmentation from short axis images and independently left and right atria segmentation from long axis images and validate its performance on large set of more than 1700 normal subjects. We then fully automatically quantified strain parameters for a subset of patients to characterize myocardial deformation, analyzing differences associated with age, gender, and height. This research has been conducted using the UK Biobank Resource1 (access application 2964).Methods
Short-axis (SAX) and long-axis (LAX) CMR CINE bSSFP available from the UK Biobank Resource 1 had been acquired using a standard protocol 2 (8 mm slice thickness, spatial resolution between 1.8-2.1 x 1.8-2.1 mm, and 31 ms temporal resolution) at 1.5 T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Manually annotated ground truth for the end-systolic (ES) and end-diastolic (ED) phases by an expert observer was available from the UK Biobank Resource 1.The deep neural networks for image segmentation were designed based on the U-net architecture 3 using 5 densely connected blocks 4 in the encoding and the decoding part of the network, respectively. For the SAX stack, a 4-class model was trained to jointly segment the left-ventricular (LV) blood pool, myocardium and the right ventricle (RV), with an additional class for the background. Data from 3000 subjects was used for training (10% reserved for validation) and 1719 unseen cases for testing, which amounts to 22640 individual SAX images. In LAX, a four-chamber view was considered for segmenting the atria. Two binary models were independently trained to segment the left (LA) and right atria (RA) and the background. 2809 cases were used for training of the LA and 2756 for the right atrium RA, while 2000 cases were reserved for testing. The results on the testing data were compared with the ground truth segmentation.
Smooth contours were automatically extracted from the probabilistic results of the neural nets and propagated to all image frames. A dense temporal deformation field was computed by inverse-consistent deformable registration 5. Based on this, the time-varying Lagrangian strain tensor was computed for the LV for a random subset of 460 healthy subjects from the testing set 6. The strain tensors were projected in cylindrical coordinates centered with respect to the LV axes, and global radial, circumferential and longitudinal strain magnitude curves (GRS, GCS, and GLS) were computed for each patient. The average strain curves were analyzed for patient subsets divided according to age, gender, height and body-mass index (BMI). Subjects with missing information were excluded for the subgroup analysis. The maximum strain magnitude was computed at ES and a middle diastolic frame was considered half-way between the ES frame and the end of the series to assess diastolic function. Statistical significance was determined using the T-test.
Results
Table 1 shows the dice scores obtained after deep-learning segmentation on the testing set for each segmented cardiac structure in short and long axis (LV 96.2%, myocardium 89.4%, RV 92.9%, LA 92.8%, RA 94.7%). Figure 1 shows the segmented contours for the LV, RV and myocardium in short and long axis, and strain curves for an example test dataset. Figure 2 shows the average GRS, GCS, GLS magnitudes over time (±SD) for the entire patient population, as well as for subgroups separated by age and gender. Table 2 shows the significant differences between patient subgroups divided by age, gender, and height. No significant differences were found in connection to BMI.Discussion
The performance of the machine-learning segmentation algorithm is similar or slightly better compared to the state-of-the-art 7. The reported strain values fall under standard reported values for normal subjects 8 and are in agreement with similar findings regarding age and gender differences 9,10 . While there was a significant difference in strain magnitude by gender, we also found a small but significant difference in strain magnitude associated to overall height or body size, which could party account for the gender differences. Radial strain magnitude was significantly larger for females, younger and shorter populations, both and ES and mid-diastole. While the end-diastolic circumferential and longitudinal strains were similar for the younger and older age groups, the mid-diastolic strain was significantly lower for subjects older than 60. This suggests that this older population may show signs of diastolic disfunction. While no significant differences were found in connection to BMI, we mention that the study population was generally healthy and very few patients were underweight or obese.Conclusion
In conclusion, we showed that we were able to reliably perform fully automatic ventricular volume quantification from short-axis CINE MRI, followed by left-ventricular strain quantification. Deep-learning powered algorithms enable the automatic quantification of large subject cohorts, which can consolidate insights in cardiac anatomy and physiology and potentially reduce the time routinely dedicated to data processing.Acknowledgements
The concepts and information presented in this paper are based on research results that are not commercially available.References
- Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. 12, e1001779 (2015).
- Petersen, S.E. et al. UK Biobank’s cardiovascular magnetic resonance protocol. 18, 8 (2015).
- Ronneberger, O., Fischer, P. & Brox, T. U-net: Convolutional networks for biomedical image segmentation. in International Conference on Medical image computing and computer-assisted intervention 234-241 (Springer, 2015).
- Huang, G., Liu, Z., Van Der Maaten, L. & Weinberger, K.Q. Densely connected convolutional networks. in Proceedings of the IEEE conference on computer vision and pattern recognition 4700-4708 (2017).
- Guetter, C., Xue, H., Chefd'Hotel, C. & Guehring, J. Efficient symmetric and inverse-consistent deformable registration through interleaved optimization. in 2011 IEEE international symposium on biomedical imaging: from nano to macro 590-593 (IEEE, 2011).
- Jolly, M.-P. et al. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. 19, 59 (2017).
- Bai, W. et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. 20, 65 (2018).
- Lamacie, M.M. et al. Quantification of global myocardial function by cine MRI deformable registration-based analysis: Comparison with MR feature tracking and speckle-tracking echocardiography. 27, 1404-1415 (2017).
- Cheng, S., Fernandes, V. R., Bluemke, D. A., McClelland, R. L., Kronmal, R. A., & Lima, J. A. (2009). Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circulation: Cardiovascular Imaging, 2(3), 191-198.
- Lawton, J. S., Cupps, B. P., Knutsen, A. K., Ma, N., Brady, B. D., Reynolds, L. M., & Pasque, M. K. (2011). Magnetic resonance imaging detects significant sex differences in human myocardial strain. Biomedical engineering online, 10(1), 76.
Figures
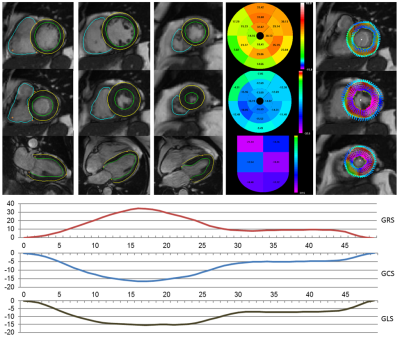
Figure 1:
Example test case. Top (left to right): automatic contours at ED and ES for
basal, mid and apical slices, 2CH ED and 4CH ED and ES; bullseye plot for
radial, circumferential and longitudinal strain at ES; overlaid contour
deformation magnitude and strain for ES frames. Bottom: Global radial, circumferential, and
longitudinal (GLS) strain magnitudes per frame.
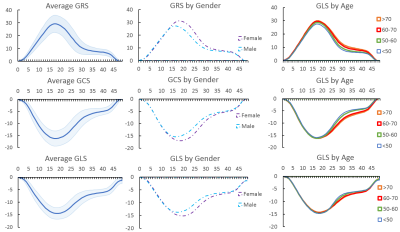
Figure 2: Top to Bottom: Global radial, circumferential, and longitudinal strain magnitude curves per time frame for the entire subset of 460 test subjects (highlighted area shows ±SD) and for subgroups divided by gender and age.
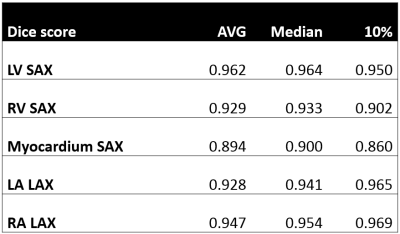
Table 1:
Dice score results after deep-learning segmentation for the segmented
structures: LV blood pool (SAX), RV blood pool (SAX), LV myocardium (SAX), LA
(LAX), RA (LAX). The average, median, and the 10th (worst) are shown
for the test set.
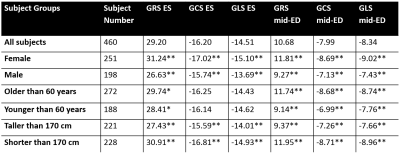
Table 2:
Systolic and diastolic GRS, GCS, and GLS magnitude for the subject population
and subgroups defined by gender, age, and height. Significant differences are
marked as ** p<0.001 and * p<0.05.