0770
A Multi-Scale Variational Neural Network for accelerating bright- and black-blood 3D whole-heart MRI in patients with congenital heart disease1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3Guy’s and St Thomas’ Hospital, NHS Foundation Trust, London, United Kingdom, 4Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
Bright- and black-blood MRI sequences provide complementary diagnostic information in patients with congenital heart disease (CHD). A free-breathing 3D whole-heart sequence (MTC-BOOST) has been recently proposed for contrast-free simultaneous bright- and black-blood MRI, demonstrating high-quality depiction of arterial and venous structures. However, high-resolution fully-sampled MTC-BOOST acquisitions require long scan times of ~12min. Here we propose a joint Multi-Scale Variational Neural Network (MS-VNN) which enables the acquisition of high-quality bright- and black blood MTC-BOOST images in ~2-4 minutes, and their joint reconstruction in ~20s. The technique is compared with Compressed-Sensing reconstruction for 5x acceleration, in CHD patients.
INTRODUCTION
Congenital heart disease (CHD) is one of the most common types of birth defect. CHD can affect different cardiac structures, such as the aorta, the pulmonary veins, as well as the atrial and ventricular septum. Bright- and black-blood cardiovascular MRI plays an important role in both the diagnosis and the follow-up of patients with CHD. Bright-blood acquisitions are performed for the visualization of the great vessels and of the course of the coronary arteries and veins, while black-blood images are preferred for the depiction of small vessels.A free-breathing motion-compensated 3D whole-heart MTC-BOOST1 prototype sequence has been recently proposed for non-contrast enhanced simultaneous bright- and black-blood MR, demonstrating high-quality delineation of arterial and venous structures. MTC-BOOST (Fig. 1A) alternates the acquisition of a Magnetization Transfer (MT)-prepared inversion recovery (IR) pulse (MTC-IR, odd heartbeats) and MT-preparation solely (MTC, even heartbeats), which are combined in a phase-sensitive IR (PSIR)2 reconstruction to obtain a third, complementary, and fully co-registered black blood volume. 2D image navigators (iNAVS) are used with this approach to correct for translational respiratory motion and achieve 100% respiratory scan efficiency3 in a predictable scan time. However, high-resolution fully-sampled MTC-BOOST still requires long acquisition times of ~12min.
In this work, we propose a novel joint Multi-Scale Variational Neural Network (MS-VNN) to enable the acquisition of high-resolution bright- and black blood MTC-BOOST images in ~2-4 minutes, and their joint reconstruction in ~20s. The proposed MS-VNN technique is evaluated in CHD patients and compared with iterative SENSE (it-SENSE)4, Compressed-Sensing (CS)5 and conventional VNN6 reconstruction for 5x acceleration.
METHODS
Data acquisition: MTC-BOOST was acquired on 36 patients with CHD on a 1.5T system (MAGNETOM Aera, Siemens Healthcare). Imaging parameters included: resolution 1.4x1.4x2.8mm (reconstructed with 1.4mm3 isotropic), flip-angle 90o, TE/TR 1.5/3.2ms, MT-preparation: 15 Gaussian pulses, flip-angle 800o, frequency offset 3000Hz, duration 20.5ms. Acquisitions were performed fully-sampled and with 5x acceleration (for 18 of the 36 patients) using a variable-density Cartesian spiral-like trajectory7.MS-VNN Architecture: Translational motion corrected undersampled k-space MTC-IR and MTC bright-blood data and corresponding coil sensitivity maps are stacked in the channel dimension and provided as input to the MS-VNN. The network consists of 10 stages, in each of which the initial reconstructions are updated according to the variational scheme shown in Fig.1B. A first path enforces data consistency to the motion corrected undersampled k-space data. The second path is a regularization operator that applies two parallel sets of real-valued filter kernels and corresponding activation functions to the magnitude and phase of the complexed-valued MTC-IR and MTC bright-blood images. For the magnitude image, a multi-scale approach is applied with 3 parallel sets of 11x11, 5x5 and 1x1 filter kernels. For the phase images, 1 set of 11x11 filter kernels are applied. The root mean-squared error was used as loss function.
MS-VNN Training: All parameters of this formulation, including the prior model defined by filter kernels, activation functions and data term weights, are learned during an offline training procedure. The network was trained on 2D complex-valued images obtained from retrospectively undersampled 5-fold 3D MTC-IR and MTC data of 18 patients (4320 images). During the training procedure, the output of the MS-VNN is compared to the corresponding fully-sampled reference images. Training is performed with retrospective undersampling data to ensure that the effect of respiratory and cardiac motion in both input and output images is identical.
Experiments: Prospectively 5x undersampled data of 18 patients (not included in the training) were used to evaluate the framework. The proposed approach was compared to it-SENSE4, Wavelet-based CS reconstruction5 and conventional VNN6 reconstruction. The undersampled reconstructions were also compared against an additional fully-sampled acquisition in terms of diagnostic image quality.
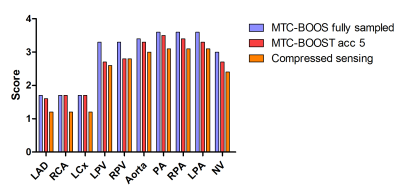
Data analysis: Diagnostic quality of the coronary arteries, pulmonary veins, aorta, pulmonary artery and neck vessels was assessed by one cardiologist, blinded to the acquisition and reconstruction method, using a 4-point Likert scale (1: non-diagnostic to excellent: 4 image quality). Analysis was performed for all subjects and for 5x CS, proposed 5x MS-VNN and fully-sampled datasets.
RESULTS
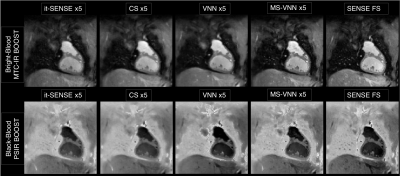
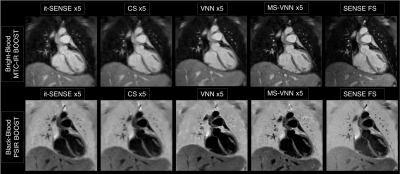
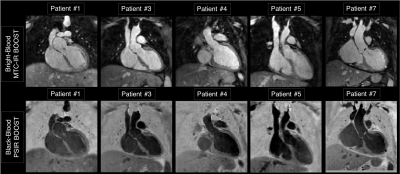
Average scan time (m:s) was 12:55 for the fully-sampled acquisition and 2:52 for an acceleration of 5x. As shown if Figs. 2 and 3 the proposed framework results in higher image quality than it-SENSE, CS and conventional VNN for both bright- and black-blood images. Consistent high image quality was observed throughout the cohort with the proposed MS-VNN (Fig. 4) for 5 representative patients. Average reconstruction time was ~10 minutes for CS and ~20 seconds for the proposed framework. Image quality score are presented in Fig. 5; demonstrating that 5-fold accelerated MTC-BOOST sequence combined with an MS-VNN reconstruction provides better image quality than CS and its overall image quality is comparable to fully-sampled MTC-BOOST acquisition.CONCLUSION
The proposed accelerated MTC-BOOST and MS-VNN framework provides high‐resolution, motion‐compensated non-contrast enhanced 3D whole-heart bright- and black-blood images, from a single acquisition of ~3min. The proposed approach offers visualization of the cardiac anatomy, including both the arterial and the venous systems. This approach has shown improved image quality with respect to it-SENSE, CS and conventional VNN reconstructions, offering high-resolution images, no need for tuning of regularization parameters, and extremely fast reconstruction time of ~20s, promising easy integration into clinical workflow.Acknowledgements
This work was supported by EPSRC (EP/P001009, EP/P007619, EP/P032311/1) and Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Ginami G et al. Non‐contrast enhanced simultaneous 3D whole‐heart bright‐blood pulmonary veins visualization and black‐blood quantification of atrial wall thickness. Magn Reson Med. 2019;81:1066–1079.
2. Kellman P et al., Phase‐sensitive inversion recovery for detecting myocardial infarction using gadolinium‐delayed hyperenhancement. Magn Reson Med. 2002;47:372-83.
3. Henningsson M, et al. Prospective respiratory motion correction for coronary MR angiography using a 2D image navigator. Magn Reson Med. 2013;69:486- 494.
4. Pruessmann KP, et al. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46:638 – 651.
5. Lustig M, et al. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195.
6. Hammernik K, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018;79:3055-3071.
7. Prieto C, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Res Imag. 2015;41:738-746.
Figures
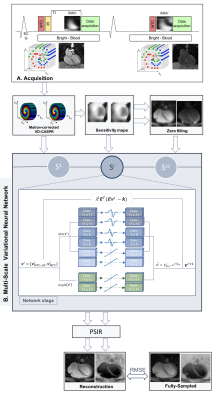
Figure 1 - Acquisition: Data were acquired using a 5-fold accelerated MTC-BOOST sequence1. MS-VNN Architecture: The MS-VNN consists in a data-consistency proximal term and in two parallel sets of real-valued filter kernels and corresponding activation functions to the magnitude and phase of the complex-valued images. For the magnitude image a multi-scale approach is applied with 3 parallel sets of 11x11, 5x5 and 1x1 filter kernels. The output MTC-IR and MTC bright-blood images are combined in a PSIR-like reconstruction in order to obtain a fully co-registered black-blood volume.