0765
Dual-Tuned Optically Controlled On-Coil Switch-Mode Amplifier
Natalia Gudino1, Stephen J Dodd1, Steve Li2, and Jeff H Duyn1
1LFMI, NINDS, National Institutes of Health, Bethesda, MD, United States, 2MIB, NIMH, National Institutes of Health, Bethesda, MD, United States
1LFMI, NINDS, National Institutes of Health, Bethesda, MD, United States, 2MIB, NIMH, National Institutes of Health, Bethesda, MD, United States
Synopsis
Optically controlled on-coil amplifiers have been presented for the practical implementation of pTx system at ultra-high field MRI. We present a new prototype that can transmit power to excite 1H and X-nuclei. We show bench and MRI data with a dual-tuned on-coil amplifier implementation for 1H and 31P excitation at 7T. We expect this technology can allow a simpler and more versatile implementation of a multinuclear multichannel hardware compared to the traditional multinuclear setup based on 50 Ω broadband voltage mode amplification.
Introduction
Human multinuclear studies at high field have the potential to become powerful diagnostic tools. Dual-tuned multichannel excitation and reception have been proposed to increased SNR and B1+ homogeneity in multinuclear imaging1,2. To simplify the implementation of multinuclear multichannel hardware, we present an optically controlled dual-tuned on-coil current-mode amplifier for 1H and X-nuclei excitation. On-coil amplification has been proposed for the practical implementation of 1H parallel transmit (pTx) systems3-5. We expect this new prototype will allow a more flexible multinuclear multichannel Tx hardware by eliminating the need of multiple cable traps, matching networks and coaxial connections needed at each resonance frequency.Methods
The dual-tuned amplifier received the optical carrier signals through a broadband optical RX interface designed previously to control on-coil amplifiers for 1H excitation4,5, the input circuitry for the preamplification and Current-Mode Class-D (CMCD) amplification stage6 were designed to maximize the gate-source voltage to fully switched ON the FETs in both stages at 1H and X-nuclei frequencies through dual-resonance LC networks (Fig. 1). For this implementation the networks were tuned for 1H (297.2 MHz) and 31P (120.3 MHz) excitation. Initial network component values were selected based on SPICE simulations (Altium Designer). A path for harmonics currents, generated from the switch-mode amplification, was provided by an output filter. The dual-resonance amplifier was connected directly (not 50 Ω impedance matching) to a dual-tuned loop. Proton and phosphorus carrier signals were connected to the input ports of a hybrid combiner the output of which was connected to the RF signal input of the optical interface box 4,5. Carrier signals were transmitted optically (not simultaneously) through a single fiber to the dual-tuned amplifier. Coil current amplitude and harmonic content was measured for both excitation frequencies with a calibrated probe coupled to the Tx coil and connected to a high-speed oscilloscope (2.5 GHz Infinium, Keysight Technologies). The performance of the dual-tuned on-coil amplifier was compared to a previous 1H-tuned on-coil prototype. A preliminary multinuclear MR experiment was performed in a 7 T MRI scanner (Siemens, Erlangen). Carrier signals from the scanner control were connected to the in-house optically interface located in the scanner electronics room. Multinuclear excitation was performed with the new amplifier and Tx coil loaded with a 31P rich solution (50 mM potassium phosphate). Proton and phosphorous signals were detected with surface loops tuned to the corresponding frequencies (120.3 MHz and 297.2 MHz). The Rx coils were connected to a 31P /1H interface box (Quality Electrodynamics, Ohio) plugged to the patient table in the 7 T scanner. A 1H localization image was acquired (5 ms TE, 20 ms TR, 192 x 192 matrix size and 8 mm slice thickness) after which spectroscopy data was acquired with a Chemical Shift Imaging (CSI) sequence (0.5 us RF hard pulse, 3 s TR, 16 x 16 matrix size, 200 mm x 200 mm FOV, 10 average).Results
Figure 2 shows simulated frequency response of the dual-resonance LC network (CMCD gate circuit) by sweeping a single component value at a time. Figure 3 shows the tuning of the dual-tuned, not 50 Ω matched, loop and corresponding load impedance for both frequencies. Coil current vs bias voltage of the amplifier power stage (VDD) for both nuclei is shown in Figure 4. In this setup, maximum power delivered to the coil was 46 W and 81 W for 31P and 1H respectively. Total harmonic distortion (THD) values, estimated from the FFT of the coil current at both frequencies, were THD ~ 1.2 % and THD~4.8 % for the 1H and 31P current respectively. No degradation in performance was observed for 1H excitation as shown from the power (delivered to the coil) measurement performed with the dual-tuned amplifier and a single-tuned 1H prototype (Fig. 4). Figure 5 shows the result of the multi-resonant MRI experiment, 31P peak (left), CSI image (center) and metabolite image (right) were successfully obtained with this preliminary setup.Discussion
The dual-tuned on-coil Tx setup allows to have multinuclear excitation without the need of additional matching networks, cable traps, and coaxial connections. Therefore, this approach can simplify the implementation of a multinuclear multichannel setup compared to those built based on 50 Ω broadband voltage amplifiers typically found in MRI systems with multinuclear capability. We presented preliminary data for 31P and 1H excitation, however the dual-tuned amplifier can be tuned to excite other X-nuclei (23Na, 13C, 19F etc.). In addition, the design of the gate circuit could be extended to allow more than 2 nuclei excitation. In this work we performed 31P and 1H excitation through a single dual-tuned Tx coil, however the design can allow the combination of the amplifier with nested coils as presented elsewhere to improve efficiency2. Finally, in a 1H pTx system built with this technology each channel can be simply “transformed” by the control to excite a different nucleus as necessary for different applications. Therefore, we consider this technology will allow the implementation of a more versatile Tx hardware to be able to excite both 1H and X-nuclei.Acknowledgements
References
1- Ianniello C, et al. Magn Reson Med. 2019 82(4):1566-1575. 2- Brown R, et al. Neuroimage. 2016 124(Pt A):602-611. 3- Gudino N, et al. Magn Reson Med. 2013 70:276-89. 4- Gudino N, et al. Magn Reson Med. 2016 76:340-9. 5- Gudino N, et al. Magn Reson Med. 2018 79(5):2833-2841. 6- Kobayashi H, et al. IEEE Trans Microwave Theory Tech 2001;49:2480–2485.Figures
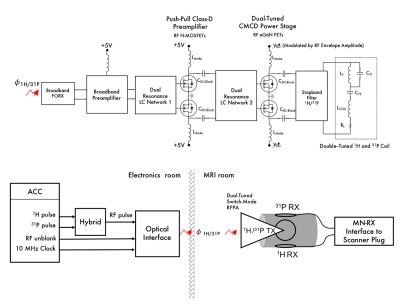
Figure 1: Schematic diagram of dual-tuned optically controlled on-coil switch-mode amplifier (top) and diagram of dual-tuned on-coil Tx setup and single-tuned Rx coils connected to scanner patient table (bottom).
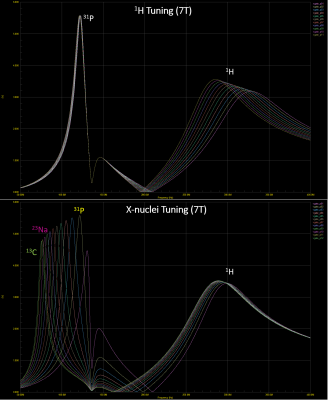
Figure 2: AC response of the gate voltage at CMCD stage during tuning of the upper frequency with a single component (top) and during tuning of the lower frequency with a single but different component of the input LC network.
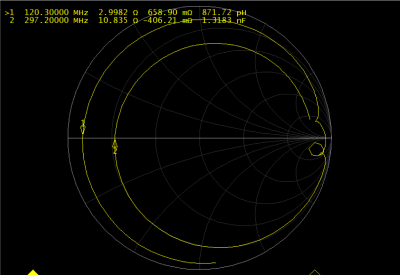
Figure 3: Tuning of the Tx surface loop for 1H and 31P driven by the on-coil amplifier. Load impedance is marked at each frequency.
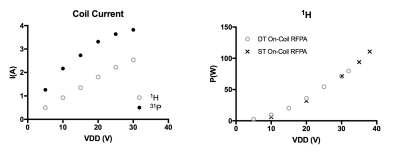
Figure 4: Transmit coil current for both nuclei (left) and power delivered to the coil for 1H excitation (right) with dual-tuned amplifier (circle) and a 1H only prototype (cross).
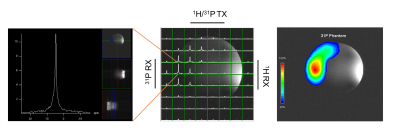
Figure 5: 7 T multinuclear MRI experiment. 31P peak (left), CSI image with overlapped 31P spectroscopy map (center), and metabolite image (right).