0760
Magnetic resonance imaging with direct optical detection1Niels Bohr Institute, University of Copenhagen, Copenhagen, Denmark, 2Department of Health Technology, Technical University of Denmark, Kgs. Lyngby, Denmark
Synopsis
A new approach to MRI detection is reported, where an optomechanical transducer directly converts and amplifies the MR signal to amplitude modulation of laser light. The transducer, which is simultaneously a capacitor and an optical cavity, is connected directly in parallel to the receive coil. The mechanical Q-factor of the transducer is 1500, providing a 3 dB bandwidth of 1 kHz. We show here for the first time that this technology can be used to directly acquire a 13C MRI at 3T (32.13 MHz), and reconstruct it using the standard pipeline of a commercial MR scanner.
Introduction
MRI hardware has evolved greatly over the last years. The introduction of receive-only coil arrays can arguably be considered one of the advances that most improved image quality1. However, the MR signal detection at its fundamental level has not changed importantly over that time: a time-varying magnetic field induces a voltage on a coil closely coupled to the sample, and that voltage is amplified and digitized.The improvement and miniaturization of the electronic components have led to coil arrays with very high number of channels. Further improvements in this direction run into problems caused by the high number of electrical signals and cables that need to be handled in the close vicinity of the patient. Many of these problems can be minimized (if not completely removed) by the use of optical technology on both the detection and signal communication processes. Optical fibers are non-metallic, insensitive to high-magnetic fields, immune to electrical interference and safer for the patient than standard electrical cables.
Converting the MR signal into the optical domain after detection has previously been reported2. In this work, we go a step further and propose to directly detect and up-convert the MR signal into an optical carrier. This allows displacing the preamplification electronics away from the coils and the patient. The approach used here is based on an optomechanical transducer, with a natural mechanical resonance at 1.4 MHz3,4.
Materials and Methods
Detection PrincipleThe transducer consists of a freely suspended membrane that acts simultaneously as one side of a capacitor and one mirror in an optical cavity5. An MR signal induces a voltage across the coil terminals that force a buildup charge on the capacitor. This process produces a force on the membrane and makes it move, which in turn changes the capacitance and consequently the charges that produce the motion. This creates a parametric coupling between the mechanical and electrical resonances. The sensitivity of the electro-mechanical transduction is optimal when the membrane is driven at its natural resonance frequency. For the case proposed here, this means that a biasing RF signal, whose frequency is the difference between the Larmor precession and the membrane resonance (32.13 - 1.4 MHz = 30.73 Mhz) has to be applied.
Transducer Properties
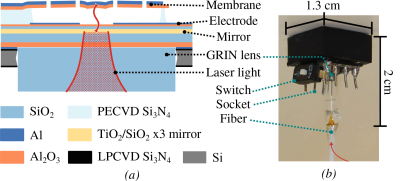
The connection between the transducer and the electrical circuit was made via wire-bonding the membrane to an 8-pin integrated circuit socket (Figure 1). Because the transducer needs vacuum to operate (to enhance the mechanical quality factor), the assembly was placed inside an MR compatible vacuum chamber made of glass-fiber that could hold a pressure below 1 × 10−3 mbar. The transducer chip and the rest of the circuit were then connected with a short RF cable via a vacuum feedthrough. For this kind of transducer, the mechanical contribution to the intrinsic noise temperature has been shown to be 113 K, while the optical noise is 90 K, and both add to a total intrinsic transducer noise of 210 K. This is equivalent to a noise-figure of 2.33 dB5.
MR Experiments
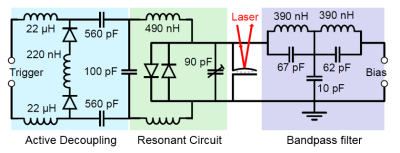
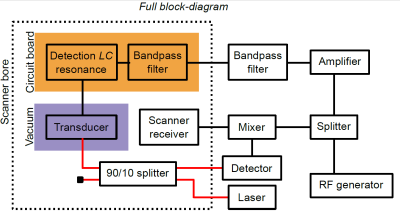
MR experiments were performed on a 3T scanner (GE Healthcare, USA), using a spherical (38 mm diameter) 13C-enriched bicarbonate phantom. A CSI sequence (16x16 acquired points, TR=75ms, FOV= 80 mm x 80 mm x 100 mm) was used. The coil used for detection was a custom made, spiral surface coil (50 mm outer diameter), tuned using two segmenting capacitors. One of the segmenting capacitors was used to implement active decoupling, in a regular fashion. The transducer was also connected to a bandpass filter, which allowed to bias it with the RF constant wave at 30.73 MHz. The circuit schematic is shown in Figure 2. The detection architecture is shown in Figure 3. The output of the optical detector is down-converted to the frequency of the mechanical resonance (1.4 MHz) and could be used to reconstruct the MR image. However, and just for convenience, we mixed that signal back to the Larmor frequency and fed it to the scanner receiver, allowing the image to be reconstructed by the dedicated scanner postprocessing software.
Results and Discussion
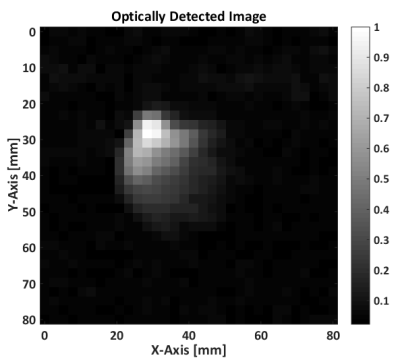
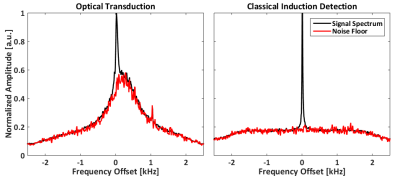
Figure 4 shows the MRI measured using the described optical detection. Figure 5 shows the averaged spectrum over the whole FOV using the optical setup (left), and compared to a similar measurement using standard detection. The single frequency peak of the bicarbonate signal can be seen clearly in both acquisitions, but their noise floor is very different. The noise floor of the optically detected signal is compatible with the mechanical membrane spectral response, following a Lorentzian distribution.Conclusion
We propose a setup for direct optical detection in MRI, and successfully implement it in a commercial MR scanner. The optically detected spectrum shows an SNR comparable to the standard detection, but still lower. However, the overall performance of the optical detection can be further enhanced with straightforward improvements (e.g. lowering the capacitance in parallel to the transducer, optimization of the optical cavity, higher bias…). The RF bias signal can be potentially applied wirelessly, therefore providing a setup with no electrical connections. Such a design would allow for a new generation of highly dense arrays, where no electrical cables are needed in the near environment of the patient.Acknowledgements
This work was supported by funding from the European Union’s Horizon 2020 research and innovation program under grant agreement 732894 (HOT) and 722923 (OMT); European Research Council (Q-CEOM 638765, QUANTUM-N 787520,EMOT 768491); Innovation Fund Denmark (Qubiz 5150-00004B); Danish Council for Independent Research (4002-00060,4005-00531); and Danish National Research Foundation (DNRF124 HYPERMAG, DNRF139 Hy-Q).References
1. Roemer PB, Edelstein WA, Hayes CE. The NMR Phased Array. MRM. 1990;225:192-225.
2. Memis OG, Eryaman Y, Aytur O, Atalar E. Miniaturized fiber-optic transmission system for MRI signals. MRM. 2008;59(1):165-173. doi:10.1002/mrm.21462.
3. Bagci T, Simonsen A, Schmid S, et al. Optical detection of radio waves through a nanomechanical transducer. Nature. 2014;507(7490):81-85. doi:10.1038/nature13029.
4. Takeda K, Nagasaka K, Noguchi A, et al. Electro-mechano-optical detection of nuclear magnetic resonance. Optica. 2018;5(2):152. doi:10.1364/OPTICA.5.000152.
5. Simonsen A, Saarinen SA, Sanchez JD, Ardenkjær-Larsen JH, Schliesser A, Polzik ES. Sensitive optomechanical transduction of electric and magnetic signals to the optical domain. Opt Express. 2019;27(13):18561. doi:10.1364/OE.27.018561.
Figures