0729
Using glutamate-CEST to characterize water diffusion inside neurons: initial results1Molecular Imaging Research Center (MIRCen), Commissariat à l'Energie Atomique et aux Energies Alternatives (CEA), Fontenay-aux-Roses, France, 2UMR 9199, Neurodegenerative Diseases Laboratory, Centre National de la Recherche Scientifique (CNRS), Université Paris-Sud, Université Paris-Saclay, Fontenay-aux-Roses, France
Synopsis
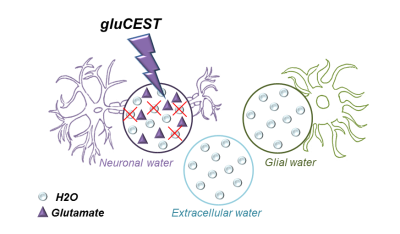
It is still unclear how diffusion properties of water differ from one compartment to another (neurons, glial cells, extracellular space…). Here we propose the idea that Chemical Exchange Saturation Transfer of Glutamate (gluCEST) may be used to specifically reduce the contribution of intraneural water to the overall signal attenuation, thus providing enhanced sensitivity to non-neuronal compartments. Acquisitions performed in two rats yields water ADC slightly but significantly higher when gluCEST is performed, supporting the idea that water diffusion is slower inside neurons.
Introduction
Diffusion MRI is a powerful tool to decipher the intricacies of tissue microstructure. However, water ubiquitously diffuses in all compartments (neurons, glial cells, extracellular space…), and it is unclear how diffusion properties differ in these compartments, which severally limits the robustness of biophysical models needed to extract quantitative microstructural parameters (e.g.1). Experimental approaches using intracerebral injection of exogenous probes such as gadolinium or 19F-labeled molecules have been proposed to gain some specificity to the extracellular space2-4. Nevertheless, such approaches are not ideal since: i) they might alter the osmotic balance and induce microstructural alterations; ii) entry inside cells cannot be ruled out; iii) they cannot be applied to Humans.Here we propose that Chemical Exchange Saturation Transfer (CEST) of endogenous metabolites may be used to specifically modulate the relative contribution of water in the different compartments, hence providing some compartment-specific insights into water diffusion properties. We implement that idea for glutamate, a widely acknowledged neuronal marker which can generate a strong CEST effect (gluCEST)5 thanks to fast exchange between protons of its amine group and water. By incorporating a gluCEST module within a diffusion-weighted sequence, we aim at reducing the contribution from intraneural water to the overall signal (Figure 1). Acquisitions performed in two rats reveal that water ADC is slightly but significantly lower when gluCEST is performed, i.e. water diffusion is slower inside neurons.
Methods
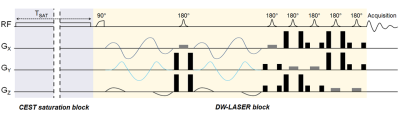
Sequence: For this initial work, we wanted to favor detection sensitivity over spatial resolution, to be able to capture subtle differences in diffusion properties. We also wanted high acquisition speed to avoid bias due to potential drifts. Therefore, we decided to measure water signal using localized diffusion-weighted magnetic resonance spectroscopy (DW-MRS) instead of DW-MRI. Briefly, a gluCEST module was positioned in front of a fully adiabatic LASER localization sequence (Figure 2). Isotropic diffusion-weighting was achieved thanks to QMAS gradients6 inserted around the first refocusing pulse7,8. The stability of water ADC with respect to varying saturation offsets was assessed in a phantom.Animal experiments: Two female Sprague-Dawley rats were anesthetized using isoflurane and scanned in an 11.7 T Bruker scanner. A volume coil was used to ensure homogenous transmit-B1 for gluCEST saturation, while a quadrature surface coil was used for reception to maximize SNR. A voxel was positioned in a glutamate-rich cortical area above the corpus callosum. For the gluCEST saturation time, we wanted to use a “short” tsat to limit potential water exchange between the different compartments, and hence avoid distribution of saturated water in all compartments that would obscure any compartment-specificity. We decided to use tsat=300 ms, which is much shorter than tsat=1 second as widely used for gluCEST. The optimal saturation parameters were found to be fsat=+3 ppm and B1=9 µT. In the end, three saturation offsets were used: +3 ppm corresponding to glutamate resonance, -3 ppm as a symmetric control, and -20 ppm as off-resonance control (the water resonance is here at 0 ppm according to the usual CEST convention). The ADC (two-point measurement at b=0.2 ms/µm² and 1.2 ms/µm²) was evaluated for each of these saturation frequencies, and the measurement was repeated ten times over an hour in each animal.
Results
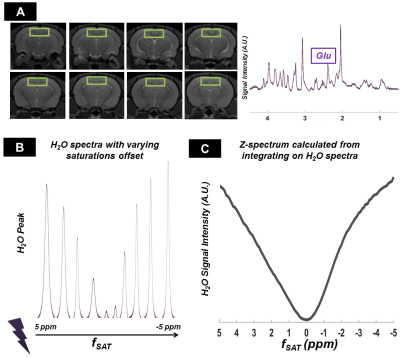
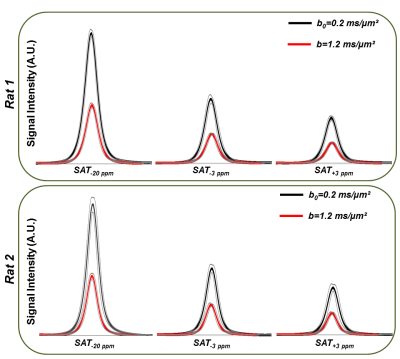
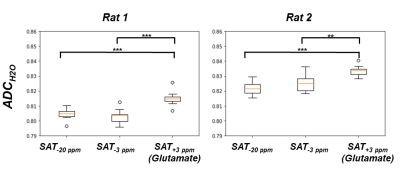
Figure 3-A shows the voxel localization together with a water-suppressed metabolite spectrum, which confirms high glutamate concentration in our voxel. Figure 3-B displays a few water peaks as measured for different fsat, and Figure 3-C exhibits the whole z-spectrum as acquired between -5 and 5 ppm by integrating water spectra acquired at different fsat in one animal. The strong gluCEST contrast can be appreciated, yielding an asymmetrical magnetization transfer ratio (MTRasym) at 3 ppm of ~15% in both animals. Figure 4 displays the mean water spectra averaged over ten acquisitions and obtained at the three aforementioned saturation offsets, with and without diffusion weighting, for the two animals. Figure 5 shows the boxplots obtained for the ten ADC values repeatedly measured in each animal for the three saturation offsets. In both cases, the ADC for fsat=3 ppm is slightly (~1%) but very significantly (p<0.005) higher than for fsat=-3 and -20 ppm, whereas there is no significant difference between the latter two.Discussion/ Conclusion
The approach presented here opens up many exciting opportunities. However, it is fundamentally limited by the necessity to generate a strong CEST contrast while keeping tsat short enough to minimize water exchange between compartments. Shorter tsat can be partly, but not fully, compensated by higher B1. Further investigations are hence required to determine optimal tsat. Interestingly, we also performed experiments at tsat=1 second (not shown), but could not observe ADC variations when glutamate was saturated, suggesting that too much inter-compartment exchange has occurred over 1 second.Here we used a spectroscopic approach, as we thought that high sensitivity would be required to detect subtle variations in ADC. However, partial volume effect from ventricles cannot be entirely ruled out when using large voxels. Future works will investigate the possibility to reduce voxel size, and to adapt the approach to DW-MRI. Besides alleviating partial volume effects, higher spatial resolution would enable measurements in highly-ordered structures, such as the corpus callosum, parallel and perpendicular to fibers.
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s FP7 and Horizon 2020 research and innovation programmes (grant agreements No 336331 and 818266). The 11.7 T MRI scanner was funded by a grant from “Investissements d'Avenir - ANR-11-INBS-0011 - NeurATRIS: A Translational Research Infrastructure for Biotherapies in Neurosciences".References
1 Jelescu, I. O., Veraart, J., Fieremans, E. & Novikov, D. S. Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue. NMR in biomedicine 29, 33-47, doi:10.1002/nbm.3450 (2016).
2 Silva, M. D. et al. Separating changes in the intra- and extracellular water apparent diffusion coefficient following focal cerebral ischemia in the rat brain. Magnetic resonance in medicine 48, 826-837, doi:10.1002/mrm.10296 (2002).
3 Kunz, N., da Silva, A. R. & Jelescu, I. O. Intra- and extra-axonal axial diffusivities in the white matter: Which one is faster? NeuroImage 181, 314-322, doi:10.1016/j.neuroimage.2018.07.020 (2018).
4 Duong, T. Q. et al. Extracellular apparent diffusion in rat brain. Magnetic resonance in medicine 45, 801-810, doi:10.1002/mrm.1108 (2001).
5 Cai, K. et al. Magnetic resonance imaging of glutamate. Nature medicine 18, 302-306, doi:10.1038/nm.2615 (2012).
6 Topgaard, D. Director orientations in lyotropic liquid crystals: diffusion MRI mapping of the Saupe order tensor. Physical chemistry chemical physics : PCCP 18, 8545-8553, doi:10.1039/c5cp07251d (2016).
7 Marchadour, C., Brouillet, E., Hantraye, P., Lebon, V. & Valette, J. Anomalous diffusion of brain metabolites evidenced by diffusion-weighted magnetic resonance spectroscopy in vivo. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 32, 2153-2160, doi:10.1038/jcbfm.2012.119 (2012).
8 Ligneul,
C. & Valette, J. Probing metabolite diffusion at ultra-short time scales in
the mouse brain using optimized oscillating gradients and
"short"-echo-time diffusion-weighted MRS. NMR in biomedicine 30, doi:10.1002/nbm.3671 (2017).
Figures