0727
Streamline tractography for 3D mapping of axon bundle organization in one MRI voxel using ultra-high resolution synchrotron radiation imaging1DTU Compute, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Danish Research Centre for Magnetic Resonance, Hvidovre, Denmark, 3Department of Neurology, Odense University Hospital, Odense, Denmark, 4X-ray Nanoprobe Group, ID16A, The European Synchrotron, Grenoble, France, 5University College London, London, United Kingdom, 6Research Laboratory for Stereology and Neuroscience, Bispebjerg University Hospital, Copenhagen NV, Denmark
Synopsis
We present an efficient image analysis pipeline that enables us to reveal white matter organization in high-resolution 3D non-MRI structural datasets, in cases where a strict image segmentation is not required nor possible. We apply the method to a synchrotron X-ray holographic tomography scan from a healthy mouse sample, and show the organization of axon bundles in a region covering parts of the corpus callosum and the cingulum. The method has a potential to improve our general understanding of white matter organization and our ability to generate realistic phantoms for validation of microstructure modelling from low-resolution diffusion MRI scans.
Introduction
Diffusion-weighted MRI (DWI) allows us to probe and model the microstructure of white matter tissues from in-vivo scans. Validation of those models is an on-going and crucial challenge for the community. The key is to work on a ground truth structural data set. Phantom-based validation is typically the go-to option, but they are in themselves simplified models of the real anatomy! Eventually, we end up studying high-resolution volumetric datasets of microstructure. In this context, a high resolution corresponds to images with enough detail, so that individual axons and other microstructures can be resolved.Making a thorough data analysis of these large volumes is required, but too time consuming and difficult to do manually. Semi- or fully automated segmentation approaches are being developed1,2. However, they are challenging to make generally applicable and work best at really high resolutions, where all structural boundaries are clearly defined. Another downside is that the high resolution typically is traded for a smaller field-of-view (FOV) and this obscures the ability to study the larger organization of tissues and the long-range behavior of white matter bundles etc.
Clearly, we need to deal with a class of white matter structural datasets, where axons are resolved almost as streamlines. Tracking and segmentation of an individual axon is in such a case almost impossible to achieve reliably. However, as we will show, it is possible to extract information about the organization in a relatively simple manner, by the use of structure tensor analysis and tractography as illustrated in Figure 1.
Methods
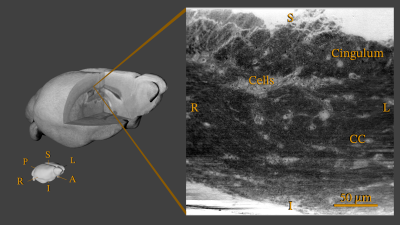
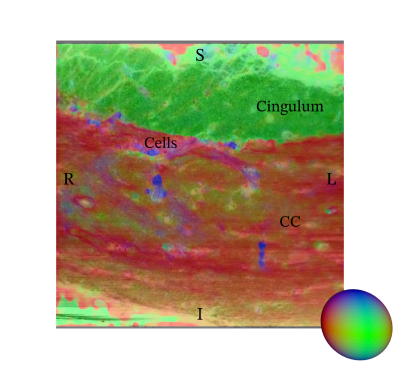
Data Acquisition: In this preliminary study, we demonstrate the method using a single healthy mouse sample. After perfusion fixation, the brain was sliced and a biopsy (approx. 2.5 x 0.7 x 0.7 mm) extracted from a region covering the splenium in Corpus Callosum (CC) and cingulum. The biopsy was stained with osmium (OsO4, 0.5%), and embedded in EPON. Imaging of the sample took place at the European Synchrotron and Radiation Facility (ESRF) at beamline ID16A using X-ray holographic nano-tomography. The obtained volume used in this study, see Figure 2, covers an extended FOV of 0.24 x 0.24 x 0.24 mm in a voxel resolution of 75 nm.Structure Tensor: The primary workhorse for the data analysis is the 3D structure tensor estimation3, here using a local Matlab implementation. In short, the image gradients in all three axis directions are measured in a small neighborhood around each voxel and collected in a 3x3 matrix. Using an Eigen-decomposition, we extract information about the local orientation. In the case of a fiber-like material such as white matter, we can estimate a clear dominant direction aligned with the main fiber orientation, see Figure 3. The concept is very similar to DTI4, but based on structural data content and not a diffusion MR signal.
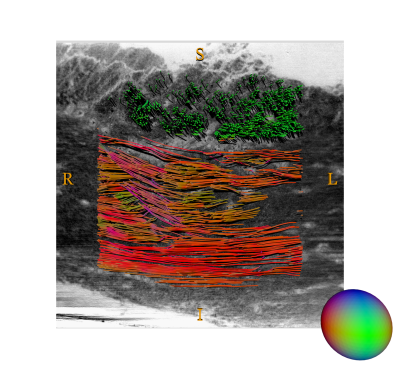
Tractography: While the structure tensor provides an estimate of the orientation information in all voxels, it does not reveal how structures are connected. That we have to probe using deterministic tractography, here using the MRTrix implementation5. The inspiration comes from DWI-based connectivity data analysis, but the application to structural data is still novel. Based on a seed point, a particle trajectory through the volume is simulated using the local main orientation for direction until some stopping mask or criteria is met, see Figure 4.
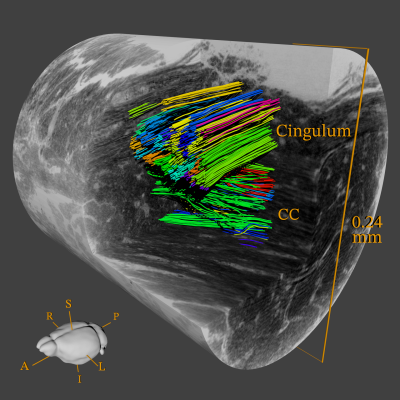
Clustering: The output of the tractography is a large number of unorganized streamlines without a direct biological interpretation. It is then beneficial to apply a streamline clustering method, which collects multiple streamlines into meaningful axonal-bundles. We use the QuickBundles method6 for its simplicity and scalability, and the result can be seen on Figure 1.
Results
Axon-bundles are clearly revealed both within the CC and cingulum. An immediate observation is that bundles trajectories are non-parallel and perform subtle bends and dispersions to move around cellular structures. An analysis on the cluster centroid trajectories, shows that bundles turn with angles up to 28.6 and 7.8 degrees in CC and cingulum respectively. Such information is valuable in the design of realistic white matter phantoms.Discussion
While we demonstrate our method on a synchrotron X-ray tomography dataset, it is in principle no hindrance to apply it to other structural and volumetric modalities. The synchrotron is a good option, as it provides relatively large FOVs with enough resolution to generate the streamline characteristic in white matter that our method targets. It is further a non-destructive technique, allowing us to cover an even larger volume with overlapping FOVs. Extensions to our work includes exploring more white matter regions of the brain and comparing healthy vs. diseased samples. More advanced approaches of both tractography and clustering can be investigated, which might be beneficial in crossing fibers regions.Conclusion
We have demonstrated an efficient image analysis pipeline based on structure tensor and tractography to investigate the 3D white matter organization. The method is ideally applied to high-resolution 3D structural datasets, in cases where a strict image segmentation is not required nor possible. It can serve to improve our general understanding of white matter organization and our ability to generate realistic phantoms for validation of microstructure modelling from low-resolution diffusion MRI scans.Acknowledgements
M. Andersson and H.M. Kjer were supported by the Capital Region Research Foundation (grant number: A5657) (PI: T. Dyrby).References
[1] Abdollahzadeh A, Belevich I, Jokitalo E, Tohka J, Sierra A. Automated 3D Axonal Morphometry of White Matter. Scientific reports. 2019;9(1):6084.
[2] Zaimi A, Wabartha M, Herman V, Antonsanti PL, Perone CS, Cohen-Adad J. AxonDeepSeg: Automatic Axon and Myelin Segmentation From Microscopy Data Using Convolutional Neural Networks. Scientific reports. 2018;8(1):3816.
[3] Khan AR, Cornea A, Leigland LA, Kohama SG, Jespersen SN, Kroenke CD. 3D Structure Tensor Analysis of Light Microscopy Data for Validating Diffusion MRI. NeuroImage. 2015;111:192-302.
[4] Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron. 2006;51(5):527-539
[5] Tournier JD, Smith RE, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A. MRtrix3: A Fast, Flexible and Open Software Framework for Medical Image Processing and Visualisation. NeuroImage. 2019;202:116-37.
[6] Garyfallidis E, Brett M, Correia MM, Williams GB, Nimmo-Smith I. QuickBundles, a Method for Tractography Simplification. Front Neurosci. 2012;6:175.
Figures