0725
Diffusion time dependence and tissue outcome in ischemic stroke1Medical Radiation Physics, Lund University, Lund, Sweden, 2Center for Medical Imaging and Physiology, Skåne University Hospital, Lund, Sweden, 3Clinical Sciences Lund, Neurology, Lund University, Lund, Sweden, 4Clinical Sciences Lund, Diagnostic Radiology, Lund University, Lund, Sweden
Synopsis
Many patients with ischemic stroke that would benefit from ‘late’ recanalization go untreated, as current imaging-based predictions of outcome are insufficiently individualized. This study investigated whether diffusion MRI (dMRI), a standard tool in stroke diagnostics, provides additional information through effects of diffusion time dependence. Results showed elevated rates of water exchange within lesions of subacute stroke patients. The absence of such exchange appeared predictive of tissue viability in the chronic stage, even in regions normally considered irreversibly injured. Information on diffusion time dependence may thus improve penumbra definitions and help identifying subjects with favorable outcome of late recanalization.
Introduction
Advances in recanalization therapy have enabled effective treatment of ischemic stroke for patients presenting before 4–5 hours1 and may benefit some patients up until 16–24 hours.2,3 Most patients that would respond to such ‘late’ recanalization go untreated, however, because predicting whether treatment will be beneficial or detrimental is challenging in individual cases. The outcome of late recanalization depends on the extent of remaining salvageable tissue (penumbra) versus the extent of already infarcted tissue risking hemorrhage (core).4 Standard estimates of these quantities using CT-perfusion and diffusion MRI (dMRI) have low accuracy,5 but the diagnostic potential of dMRI may be enhanced by going beyond conventional mapping of the apparent diffusion coefficient (ADC). In particular, ischemic stroke is associated with diffusion time dependence,6,7 which is rare in both healthy and diseased brain, that may reflect neurite beading or increased permeability. This study explored whether such time dependence may inform on tissue viability by examining subacute stroke patients longitudinally using dMRI with multiple diffusion times. Results revealed a heterogenous pattern of elevated water exchange rates within lesions that was related to the viability of tissue in the chronic stage.Methods
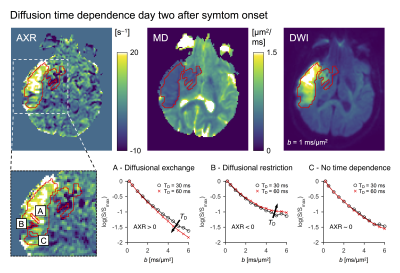
Five non-recanalized patients with ischemic stroke were scanned on an Achieva 3T system (Philips, Best, the Netherlands) at approximately two, nine and ninety days after symptom onset. dMRI data were acquired with a diffusion-weighted spin-echo sequence using two different diffusion times, TD = 30 ms and TD = 60 ms, for δ/TE/TR = 21/105/2000 ms/ms/ms, 2×2×4 mm3 resolution, and b = 0.2, 0.5, …, 6.0 ms/μm2 repeated across six directions. A DTI analysis was performed to estimate the fractional anisotropy (FA). Orientationally averaged data were analysed as in Ning et al. (2018)8 by fittingS = S0 exp(–b · MD + b2 · MK · MD2 · (1 – Γ · AXR) / 6)
to estimate the mean diffusivity (MD), the mean kurtosis (MK) and the apparent exchange rate (AXR), where the ‘exchange-weighting time’ Γ is a function of TD and δ. All data were co-registered to the first exam using Elastix.9 The lesions were defined as coherent regions of MD-hypointensity at day two, and the voxels within lesions were labelled as either ‘viable d90’ or ‘liquid d90’ based on whether their MD was below or above 1.2 μm2/ms at day ninety, respectively.
Results
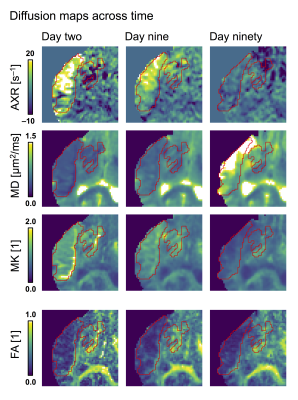
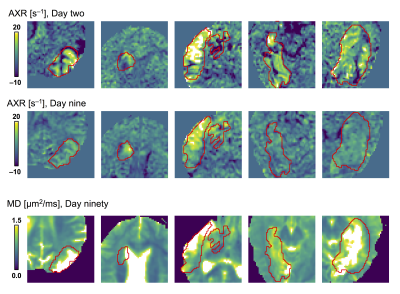
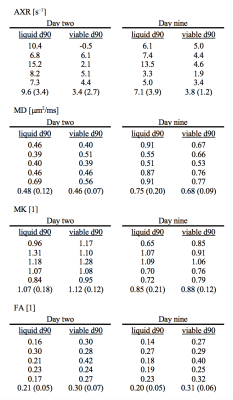
A diffusion time dependence dominated by elevated AXR values were detected at day two within lesions but not within normal-appearing tissue (Fig 2). This pattern was visible although weaker at day nine and gone by day ninety (Fig. 3). At day ninety, the MD within lesions was high in some regions, indicating liquification, while being normal-appearing in other regions (Fig. 3). The patterns of elevated AXR at day two and day nine tended to overlap with the patterns of high MD at day ninety (Fig. 4). Also, the AXR values were higher within the parts of lesions classified as ‘liquid’ at day ninety compared with parts that would be classified as ‘viable’, both at day two (9.6 ± 3.4 s–1 vs. 3.4 ± 2.7 s–1) and at day nine (7.1 ± 3.9 s–1 vs. 3.8 ± 1.2 s–1; Table 1). The MD and MK showed little relation to tissue outcome but the FA tended to be lower within parts that would be classified as ‘liquid’ (Table 1).Discussion
Ischemic stroke lesions exhibited elevated rates of water exchange in the subacute stage with a pattern that appeared predictive of tissue viability in the chronic stage, although the limited number of subjects prevented rigorous statistical analysis. An increased water exchange in ischemia could potentially be due to increased water permeability from swelling-activated volume regulatory anion channels (VRAC)10,11 or increased surface-to-volume ratio from dendritic beading.12 As this exchange appears related to tissue that is under irreversible ischemia (thus the true core) rather than tissue that may recover (Fig. 4; Table 1), its mapping using the AXR could potentially improve penumbra definitions and help identifying subjects who would have a favorable outcome of late recanalization.13,14Conclusion
Absence of diffusional exchange within ischemic stroke lesions appeared predictive of tissue viability, even in regions normally considered irreversibly injured. Future studies should explore whether this information may be used for predicting the outcome of late recanalization.Acknowledgements
We thank Philips for providing access to the pulse programming environment. The study was supported by grants from the Swedish Research Council (2016-03443).References
1. Emberson J, Lees K, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929-1935.
2. Albers G, Marks M, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708-718.
3. Nogueira R, Jadhav A, Haussen D, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2017;378:11-21.
4. Yuh W, Alexander M, Ueda T. et al. Revisiting current golden rules in managing acute ischemic stroke: evaluation of new strategies to further improve treatment selection and outcome. AJR 2017;208:32-41.
5. Kidwell C, Alger J and Saver J. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 2003;34:2729-2735.
6. Lätt J, Nilsson M, van Westen D, et al. Diffusion‐weighted MRI measurements on stroke patients reveal water‐exchange mechanisms in sub‐acute ischaemic lesions. NMR Biomed. 2009;22:619-628.
7. Baron C, Kate M, Gioia L, et al. Reduction of diffusion-weighted imaging contrast of acute ischemic stroke at short diffusion times. Stroke 2015;46:2136-2141.
8. Ning L, Nilsson M, Lasič S, et al. Cumulant expansions for measuring water exchange using diffusion MRI. J Chem Phys. 2018;148:074109.
9. Klein S, Staring M, Murphy K, et al. Elastix: a toolbox for intensity-based medical image registration. IEEE Transactions on Medical Imaging 2010;29:196-205.
10. Nilius B. Is the volume-regulated anion channel VRAC a “water-permeable” channel? Neurochemical Research 2004;29:3-8.
11. Mongin A. Volume-regulated anion channel—a frenemy within the brain. Eur J Physiol. 2016;468:421-441.
12. Murphy T, Li P, Betts K, et al. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. Journal of Neuroscience 2008;28:1756-1772.
13. Rocha M and Jovin T. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017;48:2621-2627.
14. Albers G. Late window paradox. Stroke 2018;49:768-771.
Figures
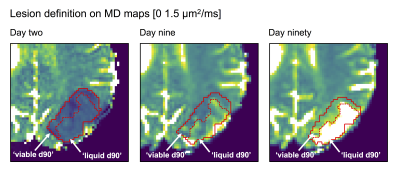
Figure 1 – Lesions were defined as coherent regions of MD-hypointensity at day two and divided between ‘viable d90’ or ‘liquid d90’ at day ninety based on whether the MD was below or above 1.2 μm2/ms, respectively. The lesion and the division between ‘viable/liquid d90’ were defined on maps from day two and day ninety, respectively, but copied to the other (coregistered) time points and manually adjusted for local changes to morphology.