0724
Validation and application of soma and neurite density imaging (SANDI) for in-vivo human brainstem nuclei atlasing1Department of Radiology, A.A. Martinos Center for Biomedical Imaging, MGH and Harvard Medical School, Boston, MA, United States, 2Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 3A.I. Virtanen Institute for Molecular Sciences, University of Eastern Finland, Kuopio, Finland
Synopsis
Despite the development of detailed in-vivo human cortical atlases, in-vivo brainstem nuclei atlasing is still at its early stages due to reduced gray-white matter MRI-contrast in the brainstem compared to the cortex. Recently, we generated an in-vivo probabilistic atlas of 16 human brainstem nuclei based on multi-contrast 7Tesla MRI. Nevertheless, to further expand the in-vivo brainstem nuclei atlas, there is an unmet need from additional MRI-contrast reflecting brainstem cytoarchitecture. We found that recently developed in-vivo Soma-And-Neurite-Density-Imaging (SANDI) provides original MRI-contrast directly related to ex-vivo brainstem nuclei cytoarchitecture, and can be used to expand the current in-vivo human brainstem nuclei atlas.
Introduction:
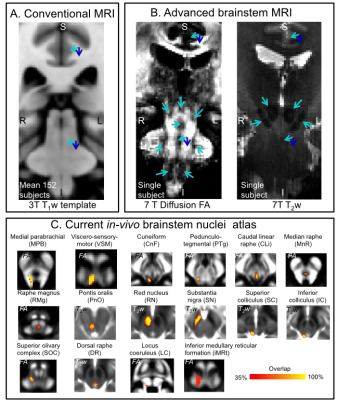
Human brainstem nuclei are more than 100 tiny gray matter regions crucially involved in a broad set of vital functions1 (e.g. arousal/sleep, autonomic, memory, affect, pain, motor, sensory, vestibular) and diseases2-5 (e.g. sleep disorders, disorders of consciousness, autonomic dysfunction, pain, Parkinson’s disease). Despite the development of detailed in-vivo atlases of the human cortex, in-vivo brainstem nuclei atlasing is still at its early stages due to reduced gray-white matter contrast in the brainstem compared to the cortex in conventional MRI (e.g. 3 Tesla T1-weighted MRI, see Figure 1A). Recently, we partially addressed this barrier by developing a multi-contrast (T2-weighted —T2w— and diffusion fractional anisotropy —FA) 7 Tesla in-vivo MRI protocol with improved contrast for brainstem nuclei (Figure 1B), and generated a probabilistic atlas of 16 brainstem nuclei in living humans6-9 (Figure 1C). Nevertheless, to further expand the in-vivo brainstem nuclei atlas, there is an unmet need from additional MRI contrast reflecting the cytoarchitecture of these gray matter regions.Purpose:
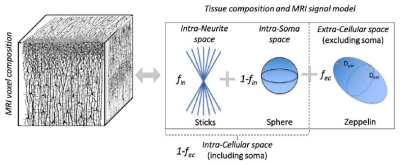
To investigate the use of recently developed Soma (i.e. cell body) And Neurite (i.e. axons and dendrites) Density Imaging10 (SANDI) (Figure 2) to expand the current in-vivo human brainstem nuclei atlas by providing original MRI contrast directly related to the brainstem nuclei cytoarchitecture.Methods:
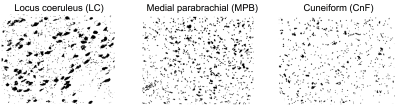
Ex-vivo histological evaluation of brainstem nuclei cytoarchitecture: We scanned and cropped images of histological Nissl stained sections1 (originally digitized with a 150x magnification) of 16 human brainstem nuclei, displaying their cell body size and distribution (Figure 3). For each brainstem nucleus, we computed the histological soma density as the number of voxels pertaining to segmented cell bodies divided by the total number of voxels in the image, and used it to validate in-vivo SANDI soma MR signal fraction (fsoma) in the brainstem.In-vivo data acquisition: We used the publicly-released multi-shell MGH Adult Diffusion Data (https://www.humanconnectome.org) acquired on 23 healthy subjects (age range: 25-35 years) under IRB-approval using a 3 Tesla Connectom scanner, a custom-made 64 channel brain coil array and parameters: 1.5mm-isotropic resolution single-shot 2D spin-echo EPI, n. slices=96, echo-time=57 ms, repetition-time=8.8s, GRAPPA-factor=3, phase-encoding direction=anterior/posterior, bandwidth=1984 Hz/pixel, b-values=[1000,3000,5000,10000,10000] s/mm2, n. diffusion-directions = [64,64,128,256,256], δ=13 ms, Δ=22 ms, 40 interspersed “b0” images (T2w, non-diffusion weighted, b-value = 0 s/mm2), total acquisition time=89’.
In-vivo data analysis: a) Preprocessing: Minimum data preprocessing included gradient-non-linearity (Matlab), motion (FreeSurfer) and eddy-current (FSL, eddy) correction. b) Computation of tensor invariants and SANDI maps: From the pre-processed data, the diffusion tensor and tensor-invariants (such as FA and average T2w) were computed (FSL, dtifit). To compute SANDI maps, the direction-averaged diffusion MRI signal was analysed as described in10. Briefly, the SANDI model (Eq. 10 in SANDI work10 and Figure 2) was fitted to the data to estimate five model parameters: soma MR apparent radius (rs), intra-neurite MR signal fraction (fin), extra-cellular MR signal fraction (fec), intra-neurite axial (Din) and extra-cellular isotropic (Dec) apparent diffusion coefficient. The intra-soma MR signal fraction was then computed as fis = 1- fin and the intra-cellular MR signal fraction fic = 1- fec. In this work we focused on the soma MR signal fraction (fsoma = fic x fis) and rs, related respectively to the cell body density and size. c) Image coregistration to sterotactic space: To map SANDI maps to the brainstem nuclei atlas, we computed the bivariate high-dimensional diffeomorphic transformations (ANTs) between sterotactic (IIT-MNI) FA/T2w templates11 and single-subject FA/T2w images using an intermediate group-based optimal bivariate template. We then applied these transformations to SANDI maps fsoma and rs. d) SANDI MRI-based evaluation of in-vivo brainstem microstructure: For each subject, we computed the mean FA, T2w, fsoma and rs across voxels within each brainstem nucleus, and then averaged these values across subjects. e) Correlation of in-vivo SANDI MRI-based and ex-vivo histological measures of brainstem microstructure: We computed the Pearson’s correlation coefficient between the in-vivo MRI group-averaged SANDI fsoma and the histological soma density of 16 brainstem nuclei.
Results and Discussion:
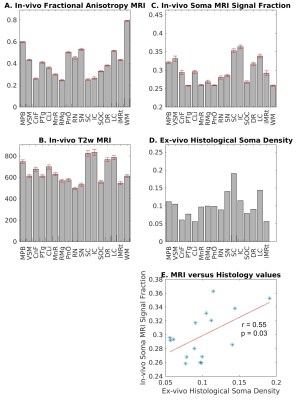
In Figure 4A-C, we show the mean ± s.e. in-vivo FA, T2w, and fsoma across subjects. Interestingly, the FA (Figure 4A) varied between about 0.25 and 0.6 within brainstem nuclei, indicating different amounts of impermeable (for a diffusion time < 20 ms12), oriented neurites within these gray matter regions. We also observed variations of T2w values across brainstem nuclei (Figure 4B), suggesting different iron contributions within these regions. Crucially, changes in in-vivo SANDI fsoma across brainstem nuclei significantly correlated (r = 0.55, p = 0.03) with variations of ex-vivo histological soma density (Figure 4C-E). This demonstrates that SANDI MRI contrast is directly related to brainstem nuclei cytoarchitecture. Conversely, in-vivo FA and T2w did not correlate with ex-vivo histological soma density (r = 0.11, p = 0.69, and r = -0.18, p = 0.51, respectively). Finally, in Figure 5, we show the high contrast provided by SANDI fsoma and rs maps for brainstem nuclei visualization.Conclusion:
We validated the use of in-vivo SANDI MRI as an original MRI contrast reflecting the cytoarchitecture of human brainstem nuclei. SANDI MRI is a promising tool to expand the current in-vivo human brainstem nuclei atlas.Acknowledgements
NIH-NIBIB K01EB019474; NIH-NIDCD R21DC015888; MGH Claflin Distinguished Scholar; Harvard Mind-Brain-Behavior Faculty Award; Engineering and Physical Sciences Research Council (EP/N018702/1, EP/G007748, EP/I027084/01, EP/L022680/1, EP/M020533/1, EP/M507970/1); NIHR-UCLH Biomedical Research Centre.References
1. Olszewski J, Baxter D, Karger S (Ed.), Cytoarchitecture of the Human Brainstem, JB Lippincott Company, Philadelphia and Montreal North America, 1954.
2. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211.
3. Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain. 2007;130(11):2770-2788.
4. Brown EN, Lydic R, Schiff ND, 2010. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638-2650.
5. Edlow BL, Haynes RL, Takahashi E, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72(6):505-523.
6. Bianciardi M, Toschi N, Edlow BE, et al. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connectivity. 2015;5(10):597-607.
7. Bianciardi M, Strong C, Toschi N, et al. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7 T MRI, Neuroimage, 2018;170:222-230.
8. Garcia Gomar MG, Strong C, Toschi N, et al. In vivo probabilistic structural atlas of the inferior colliculus, superior colliculus, medial geniculate nucleus, and lateral geniculate nucleus based on 7 Tesla MRI. Frontiers in Neuroscience. 2019;13:764.
9. Singh K, Indovina I, Augustinack JC, et al. Probabilistic atlas of the lateral parabrachial nucleus, medial parabrachial nucleus, vestibular nuclei complex and medullary viscero-sensory-motor nuclei complex in living humans from 7 Tesla MRI. bioRxiv preprint. (https://www.biorxiv.org/content/10.1101/814228v1).
10. Palombo M, Ianus A, Guerreri M, et al. SANDI: a compartment-based model for non-invasive apparent soma and neurite imaging by diffusion MRI. 2019; arXiv preprint (https://arxiv.org/abs/1907.02832).
11. Varentsova A, Zhang S, Arfanakis K., Development of a high angular resolution diffusion imaging human brain template. Neuroimage. 2014;91:177-186.
12. Yang DM, Huettner JE, Bretthorst GL, et al. Intracellular water preexchange lifetime in neurons and astrocytes. Magn Reson Med. 2018;79(3):1616-1627.
Figures