0711
Prostate cancer multiparametric MRI comparison study of 3T versus 7T: lesion detection and study design considerations1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Radiology, University of Minnesota, Minneapolis, MN, United States, 3Urology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Recent works have demonstrated the feasibility of prostate multiparametric MRI (mpMRI) at 7T with improved resolution compared to mpMRI at 3T. However, the clinical relevance of finer anatomic details versus the drawbacks of increased imaging artifacts at 7T has yet to be investigated. In this work, we conducted a retrospective, multi-reader clinical evaluation of 19 paired mpMRI studies at 3T and 7T. The primary outcome of interest was accuracy of prostate cancer detection, with image quality and artifacts as secondary outcomes.
Introduction
Recent works have described protocols for prostate multiparametric MRI (mpMRI) at 7T. These have demonstrated improved anatomic detail from increased resolution afforded by increased SNR at 7T, though with downsides of increased sensitivity to imaging artifacts. To further previous works, the goal of this study was to perform a retrospective clinical evaluation of mpMRI at 3T vs. 7T primarily in terms of prostate cancer localization. Subjective measures of image quality and artifacts were also evaluated.Methods
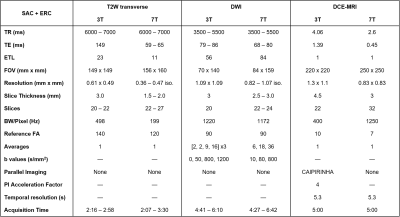
Nineteen subjects were imaged at 3T and 7T between March 2016 and October 2018 under IRB-approved protocols. Surface array coils (SACs) with and without endorectal coils (ERCs) were used at both field strengths, with the most common set-up being SAC+ERC at both (13/19 subjects). A balloon-type coil was used at 3T, and a solid two-channel coil was used at 7T as commercial ERCs do not exist at 7T. At 3T, studies were performed in accordance with PI-RADS v2 guidelines.1 At 7T, analogous studies were carried out using previously-described imaging sequences that evolved over time with hardware and methods improvements (Table 1).2-6 Briefly, T2W at 7T used a standard 2D fast spin-echo sequence with significantly increased spatial resolution to leverage the available SNR, and fat suppression was used to address the increased chemical shift artifact. Whereas DWI at 3T used 2D RF excitation to reduce echo times and imaging artifacts, these methods were not available at 7T, and thus saturation pulses were applied anterior/posterior to the prostate to achieve comparable results. Whereas DCE-MRI at 3T used an accelerated 3D T1W GRE sequence, a 3D radial UTE sequence was used to address the increased R2* relaxation.7Four radiologists retrospectively and independently reviewed the data over two separate sessions. To minimize information bias, datasets from the same subject were read in separate sessions and in random order, and readers were blinded to all clinical data. In each session, each reader read half of the 3T studies and half of the 7T studies, and completed a two-part assessment for each dataset. In the first part, readers assessed the likelihood of cancer using PI-RADS v2.1 guidelines. For each study, up to three lesions were identified, and PI-RADS 4+ lesions were demarcated on the PI-RADS reporting template. Accuracy of cancer localization was compared to findings from standard TRUS-guided sextant biopsy that were available for 13/19 subjects. For each study, the numbers of correctly or incorrectly classified sextants were summed across all four readers, then used to calculate binary measures of detection performance (Table 2a). In the second part, readers assigned a score on a five-point Likert scale to multiple image quality characteristics for the T2W, DWI, and DCE datasets separately. Anatomic visualization was also assessed for the T2W dataset (Table 2b).
Results
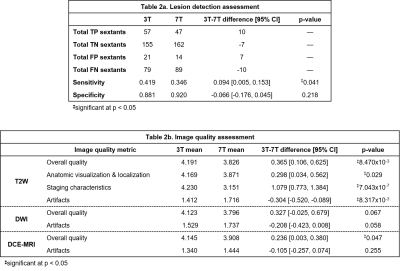
Sensitivity and specificity of the 3T and 7T datasets for sextant-wise cancer detection were compared by applying paired two-tailed t-tests to the pooled results. Readers identified more sextants harboring cancer with the 3T datasets while false-positive rates were similar, resulting in significantly higher sensitivity at 3T with no significant differences in specificity.Likert scores for image quality characteristics for the 3T and 7T datasets were compared by applying paired two-tailed t-tests to the mean scores of the four radiologists for each dataset. For T2W images, readers generally preferred the 3T datasets, in particular for staging and assessment of potential extraprostatic extension. Readers also preferred the 3T datasets for overall image quality of the DCE data (Figure 1).
Discussion
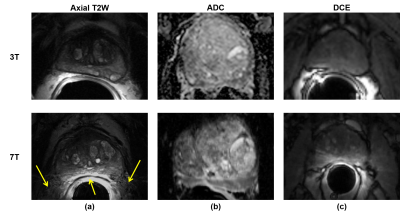
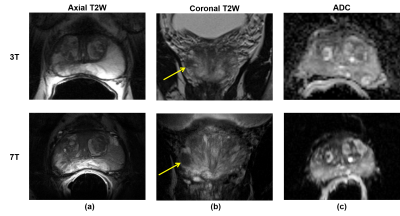
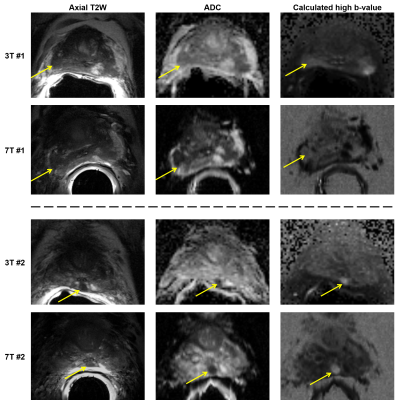
The results should be interpreted in light of two major extenuating factors. First, three of the four radiologists were unfamiliar with 7T prostate MRI and therefore were likely systematically biased in favor of the 3T images. This was further exacerbated by the use of a solid ERC for the majority of 7T studies causing worsened motion artifacts compared to the balloon ERC at 3T, as well as the fact that readers were blinded to the pairing of 3T and 7T studies. In anecdotal side-by-side comparisons of 3T-7T study pairs, it was agreed that 7T produced images with significantly more anatomic detail, though with equivocal clinical relevance and more pronounced artifacts, in particular stronger signal inhomogeneity caused by the ERC (Figure 2). Unfamiliarity with 7T likely also factored in to the missed diagnosis of biopsy-proven cancers in some cases (Figure 3). In light of these issues, we plan to focus on SAC-only imaging at 7T. Additionally, an unblinded, side-by-side clinical study comparing 3T-7T study pairs beyond a PI-RADS based evaluation may also be helpful to investigate the value of the increased anatomic detail at 7T.Additionally, evolution of the methods at 7T during the imaging portion of the study meant that the relative quality of 7T images improved over time. If data from the 6 studies conducted in 2016 were excluded from the analysis, both sensitivity of sextant-wise cancer detection and perceived quality of the DCE data would be equivalent. Demonstrated non-inferiority of cancer detection will help pave the way for clinical approval of 7T for prostate investigations in the future, and forthcoming technological developments and increased radiologist familiarity with 7T body imaging data are anticipated to improve upon the results shown here.
Acknowledgements
This work was supported by the National Institutes of Health: R01-CA1155268, P41-EB015894, T32-GM008244, TL1-TR002493, UL1-TR002494.References
1. Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019 Sep;76(3):340-351.
2. Erturk MA, Raaijmakers AJ, Adriany G, Ugurbil K, Metzger GJ. A 16-channel combined loop-dipole transceiver array for 7 Tesla body MRI. Magn Reson Med. 2017 Feb;77(2):884-894.
3. Metzger GJ, Vande Moortele PF, Snyder C, Vaughan JT, Ugurbil K. Local B1 Shimming for the Prostate at 7 Tesla. Proc Intl Soc Magn Reson Med. 2007;15:799.
4. Erturk MA, Tian J, Vande Moortele PF, Adriany G, Metzger GJ. Development and evaluation of a multichannel endorectal RF coil for prostate MRI at 7T in combination with an external surface array. J Magn Reson Imaging. 2016 Jun;43(6):1279-87.
5. Erturk MA, Metzger GJ. Prostate MRI at 7.0 Tesla Using an Actively-Tuned Endorectal Coil. Proc Intl Soc Magn Reson Med. 2016;24:3501.
6. Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001 Jul;46(1):6-12.
7. Kobayashi N, Bolan PJ, Metzger GJ. Evaluation of UTE for improved contrast enhanced DCE MRI at 7T. Proc Intl Soc Mag Reson Med. 2018;26:498.
Figures