0709
Comparing the Diagnostic Accuracy of Luminal Water Imaging versus PI-RADSv2.1 in Detection of Prostate Cancer1UBC MRI Research Centre, Vancouver, BC, Canada, 2Department of Radiology, The University of British Columbia, Vancouver, BC, Canada, 3Department of Urologic Sciences, The University of British Columbia, Vancouver, BC, Canada, 4Vancouver Prostate Centre, Vancouver, BC, Canada, 5Department of Pathology and Laboratory Medicine, The University of British Columbia, Vancouver, BC, Canada
Synopsis
Luminal water imaging (LWI) is an MRI technique that detects regions of decreased lumen volume in prostate. Recent studies on LWI have shown promising results regarding the accuracy of this technique in diagnosis of prostate cancer. However, to the best of our knowledge no study has yet compared the performance of LWI against the current clinical assessment. In this study, we perform a comparison between the diagnostic accuracy of LWI with the PI-RADSv2.1 assessment. Our results show that LWI performs similar to the PI-RADSv2.1 in the entire prostate and peripheral zone, and outperforms significantly in transition zone.
Purpose:
To compare the accuracy of detection of prostate adenocarcinoma (PCa) achieved from luminal water imaging (LWI) and mp-MRI PI-RADSv2.1.Introduction:
Luminal Water Imaging (LWI), an MRI technique that detects regions of decreased lumen volume in prostate, was first introduced1 with the purpose of detection and grading of PCa. Since then, a number of follow up studies 2-5 have been conducted to evaluate the applicability and accuracy of this technique; all with consistent and promising results. Although the results of these primary studies reveal the potential benefits of LWI in clinical prostate MRI, it is necessary to compare its performance against the current clinical assessment before proposing it as a method for population-level utilization. In this study, we perform a detailed comparison between the diagnostic accuracy of LWI and the PI-RADSv2.16 assessment performed by an experienced radiologist.Methods:
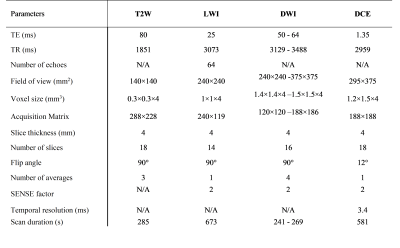
40 patients with biopsy proven PCa were examined with LWI, high spatial resolution T2-weighted (T2W), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) MRI on a 3T scanner [Philips Medical Systems, Best, The Netherlands] prior to undergoing radical prostatectomy. MRI signals were acquired with a combined endorectal/pelvic phased-array coils. Sequence parameters are presented in Table 1. From LWI data, maps of seven metrics: T2-short, T2-long, gmT2, LWF, Ashort, Along, and Ncomp were generated by fitting the signal decay curve to a multi-exponential function, as previously described1,7. Maps of these parameters were correlated to whole-mount histology following image registration.The T2W, DWI and DCE sequences were interpreted by a radiologist, with 20 years’ experience in interpreting prostate MRI, according to the current PI-RADSv2.1. The images were interpreted on a picture archiving computer system (PACS) station without knowledge of the prostate-specific antigen (PSA), PSA density or biopsy results. Each lesion given a PI-RADS score of 3 or above has localized into the sector diagram as per PI-RADSv2.1. An overall PI-RADS score of 4 or 5 was considered positive. Scores of 3 or less were considered negative. The sensitivity, specificity and area under the curve (AUC) were calculated based on the receiver operating characteristics (ROC) analysis.
ROC analysis for LWI data was performed based on logistic generalized linear mixed effect models (GLMMs), with the binary dependent variable being the malignancy status, and the predictor variable being a LWI parameter. For ROC analysis in each specific zone (peripheral zone (PZ), and transition zone (TZ), separately) the patient identifier was considered as the random intercept in the model, while in the entire prostate (including PZ, TZ, and stroma), both the patient identifier and the location were considered as the random intercepts.
ROC analysis for PI-RADSv2.1 was performed using logistic regression, with the binary dependent variable being the malignancy status, and the predictor variable being the PI-RADS score. The significance of the difference between the AUC values was tested using two-tailed t-test (MedCalc v. 19.1, Mariakerke, Belgium).
Results:
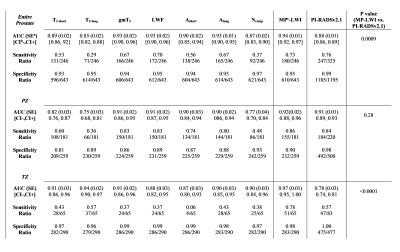
The values of AUC, sensitivity, and specificity calculated from ROC analyses are summarized in Tables 2. Among the single LWI parameters, the highest AUC was obtained for: gmT2, LWF and Along (0.93) in the entire prostate, gmT2, and LWF (0.91) in the PZ, and T2-long (0.94) in the TZ. The AUC values obtained from multi-parametric ROC analysis of LWI were equal to 0.94, 0.92 and 0.97, in the entire prostate, PZ, and TZ, respectively. The AUC values obtained from ROC analysis of PI-RADSv2.1 were all smaller, equal to 0.88, 0.91 and 0.78, in the entire prostate, PZ, and TZ, respectively, with the significant differences observed in the entire prostate, and TZ. Specificity values obtained from PI-RADSv2.1 were always higher than multi-parametric LWI (0.99, 0.98, and 1.00 vs. 0.95, 0.90, and 0.98 in the entire prostate, PZ, and TZ, respectively). Sensitivity values obtained from PI-RADSv2.1 were lower than multi-parametric LWI in the PZ and TZ (0.84 vs 0.86, and 0.57 vs. 0.78, respectively), but higher in the entire prostate (0.76 vs. 0.73).Discussion:
Comparing the diagnostic accuracies obtained from LWI and PI-RADSv2.1 assessment shows that LWI provides higher AUC values than those obtained from PI-RADSv2.1. In comparison between the specificities, PI-RADSv2.1 performs slightly better compared to LWI. However, in comparison between the sensitivities LWI outperforms considerably in TZ, and performs relatively similar in the entire prostate and PZ.Conclusion:
This study was conducted to compare the performance of LWI against the current clinical assessment in detection of PCa. Results of this study show that LWI performs equally well or better than PI-RADSv2.1 in diagnosis of PCa. The significant improvement in the AUC and sensitivity provided by LWI in TZ is of high importance, considering that TZ tumors usually pose a clinical challenge in detection and are often missed during biopsy sessions8.This technique neither requires the administration of a contrast agent, which is an inseparable component of current PI-RADSv2.1, nor suffers from the artifacts and spatial distortions commonly seen in DWI scans. Inclusion of this technique in clinical prostate MRI can potentially increase the accuracy of diagnosis of PCa, and reduce the concerns regarding the repetitive use of contrast agents.Acknowledgements
This study was supported by the Canadian Institutes of Health Research.References
1. Sabouri S, Chang S.D, Savdie R, et al. Luminal Water Imaging: A New MR Imaging T2 Mapping Technique for Prostate Cancer Diagnosis. Radiology. 2017;284(2):451-459.
2. Sabouri S, Chang S.D, Goldenberg S.L, et al. Comparing diagnostic accuracy of luminal water imaging with diffusion-weighted and dynamic contrast-enhanced MRI in prostate cancer: A quantitative MRI study. NMR Biomed. 2019;32(2):e4048.
3. Chan R.W, Lau A.Z, Detzler G, Thayalasuthan V, Nam R.K, Haider M.A. Evaluating the accuracy of multicomponent T2 parameters for luminal water imaging of the prostate with acceleration using inner-volume 3D GRASE. MRM. 2019;81(1):466-476.
4. Devine W, Giganti F, Johnston EW, et al. Simplified Luminal Water Imaging for the Detection of Prostate Cancer From Multiecho T2 MR Images. JMRI. 2019;50(3):910-917.
5. Carlin D, Orton M.R, Collins D, deSouza N.M. Probing structure of normal and malignant prostate tissue before and after radiation therapy with luminal water fraction and diffusion-weighted MRI. JMRI. 2019;50(2):619-627.
6. Turkbey B, Rosenkrantz A.B, Haider M.A, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. European Urology. 2019;76(3):340-351.
7. Sabouri S, Fazli L, Chang S.D, et al. MR measurement of luminal water in prostate gland: Quantitative correlation between MRI and histology. JMRI. 2017;46(3):861-869.
8. Lawrentschuk N, Haider M.A, Daljeet N, et al. Prostatic evasive anterior tumours: the role of magnetic resonance imaging. BJU Int 2010;105:1231–1236.