0707
Towards In Vivo Prostate Microstructure Mapping Using Diffusion-Relaxation Correlation Spectrum Imaging1Department of Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Recent work has demonstrated the ability of Diffusion-Relaxation Correlation Spectrum Imaging (DR-CSI) to quantify prostate microscopic tissue compartments (epithelium, stroma and lumen) in ex vivo prostate specimens at 3T using whole-mount digital histopathology as the reference. This study further developed DR-CSI for in vivo characterization of prostate microstructure using high resolution ex vivo DR-CSI as the reference. Consistent trends in DR-CSI signal component fraction variations in different prostate regions were found using in vivo DR-CSI across subjects, and agreed with trends on ex vivo DR-CSI.
Introduction
Microstructural MRI has the potential to improve prostate cancer (PCa) diagnosis and characterization1-3. Recently, Diffusion-Relaxation Correlation Spectrum Imaging (DR-CSI) has demonstrated the ability to quantify prostate microscopic tissue compartments (epithelium, stroma and lumen) in ex vivo prostate specimens at 3T using whole-mount digital histopathology as the ground truth4. To further develop DR-CSI as a tool for characterizing in vivo prostate microstructure, the purpose of this study was to investigate prostate DR-CSI characteristics and repeatability in human subjects. To calibrate in vivo prostate DR-CSI, ex vivo prostate DR-CSI was used as a reference.Methods
Study Design: In an IRB and biosafety approved study, 4 healthy male subjects (age: 28±2 years) were scanned using body array coils and a fresh ex vivo prostate specimen from a PCa patient was scanned using a knee coil at 3T (Prisma, Siemens). The ex vivo experimental setup and DR-CSI protocol (Figure 1a) were based on previous work4. The ex vivo DR-CSI results were compared to area fractions of microscopic tissue compartments (fepithelium, fstroma, flumen) from registered whole-mount digital histopathology (Tissue Studio 4.1, Definiens)4. The in vivo DR-CSI results were calibrated using the ex vivo DR-CSI results, which have higher signal-to-noise ratio (SNR), higher spatial resolution (1.0x1.0mm2), and sharper T2-D spectral peaks.In Vivo DR-CSI: 11 combinations of echo time (TE) and b-values were acquired in 12min (Figure 1b). Compared to ex vivo DR-CSI, the in vivo protocol had increased number of averages to maintain SNR, and included TE=180ms to accommodate longer in vivo luminal T2 relaxation time3.
DR-CSI Model: Voxel-wise T2-D spectra were reconstructed using the inverse 2D Laplace transform with non-negativity and spatial total variation constraints5. Signal component fractions (f) were computed by integrating each spectral peak in the normalized voxel-wise T2-D spectra.
Ex Vivo Analysis: Using 3D-printed patient-specific prostate molds and non-rigid registration4, ex vivo DR-CSI (fA, fB, fC) were compared to (fepithelium, fstroma, flumen) from whole-mount histopathology on a regional basis. The ex vivo T2-D peak locations and signal component fraction maps were used to define the areas under the peaks for in vivo DR-CSI.
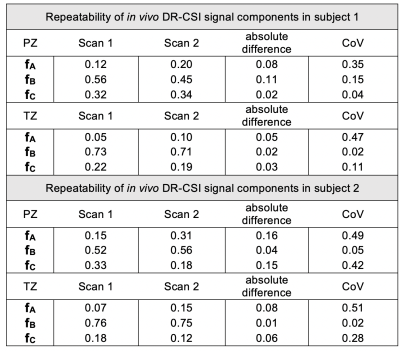
In Vivo Analysis: Rigid registration was performed among TE-b-encoded images to address motion prior to DR-CSI model fitting6. The slice-averaged T2-D spectra and corresponding (fA, fB, fC) were compared from apex to base to evaluate variations in prostate microstructure. The mean and standard deviation (SD) of (fA, fB, fC) in the transition zone (TZ) and peripheral zone (PZ) were computed for the entire prostate, across subjects. To assess repeatability of (fA,fB,fC), DR-CSI was scanned twice in the same session in two subjects, the absolute difference and coefficient of variation (CoV) were reported.
Results
Ex Vivo DR-CSI: Three distinct and stable T2-D spectral peaks were consistently resolved across slices (Figure 2). The ex vivo DR-CSI (fA, fB, fC) were in good agreement with (fepithelium, fstroma, flumen), respectively, in PCa and benign tissue regions.In Vivo DR-CSI: After comparing to an ex vivo reference case (Figure 2), the following spectral peaks were defined: peak A [T2≥80ms, D<1500 x10-6mm2/s], peak B [T2<80ms], and peak C [T2≥ 80ms, D≥700 x10-6mm2/s]. Representative T2-D spectra and signal component fraction maps are shown in Figure 3. A consistent trend of decrease in fA and fC, and increase in fB from apex to base were observed in vivo (Figure 4a), which agreed with the trend observed in ex vivo DR-CSI. The zone-specific mean±SD of (fA, fB, fC) are summarized in Figure 4b. The (fA, fB, fC) measurements in PZ and TZ from repeated acquisitions had absolute differences <0.17 (Figure 5). fA had a higher CoV than fB and fC, partly because in healthy subjects the fA (corresponding to epithelium) is relatively low.
Discussion
Ex vivo DR-CSI (fA, fB, fC) closely corresponded to (fepithelium, fstroma, flumen), respectively, indicating the ability of DR-CSI to characterize prostate microstructure. In healthy subjects, the trends in (fA, fB, fC) from prostate apex to base agree with the known increase in stroma and decrease in glandular tissues (epithelium and lumen) from apex to base7. Variations in (fA, fB, fC) for PZ, TZ, and the overall prostate agree with previous prostate microstructure findings using histopathology or MRI8-10. In comparison to ex vivo DR-CSI, in vivo DR-CSI (fA, fB, fC) were similar but also exhibited some differences, such as lower fA than ex vivo. This difference may be due to the fact that the ex vivo specimen was from a PCa patient, and thus contained more epithelium than in vivo healthy subjects.In vivo DR-CSI faces challenges such as lower spectral resolution and reduced SNR. We used the higher quality ex vivo DR-CSI results to calibrate the definition of spectral peaks for in vivo DR-CSI, and achieve consistent quantification of (fA, fB, fC). Future work to improve in vivo DR-CSI would include increasing the number of TE-b encodings while managing scan time with acceleration techniques, addressing more complex subject motion with non-rigid registration, and evaluations in PCa patients.
Conclusion
We have demonstrated that in vivo DR-CSI can characterize prostate microstructure in healthy subjects within 12min at 3T. The DR-CSI signal component fractions are consistent with prostate tissue microstructural characteristics, and might provide unique information for PCa diagnosis.Acknowledgements
This work was supported in part by Siemens Healthineers, and the Department of Radiological Sciences, David Geffen School of Medicine at UCLA.References
1. Panagiotaki E, Chan RW, Dikaios N et al. Microstructural characterization of normal and malignant human prostate tissue with vascular, extracellular, and restricted diffusion for cytometry in tumours magnetic resonance imaging. Invest Radiol 2015;50(4):218–227.
2. Chatterjee A, Watson G et al. Changes in epithelium, stroma and lumen space correlate more strongly with Gleason pattern and are stronger predictors of prostate ADC changes than cellularity metrics. Radiology 2015; (3) 751-762
3. Sabouri S, Chang SD, Savdie R, et al. Luminal Water Imaging: A New MR Imaging T2 Mapping Technique for Prostate Cancer Diagnosis. Radiology. 2017;284(2):451–459. doi:10.1148/radiol.2017161687
4. Zhang Z, Magyar C, Priester A, et al. Characterization of prostate microstructure using diffusion‐relaxation correlation spectrum imaging and comparison to digital histopathology. In: Proc 27th Annual Meeting ISMRM, Montreal; 2019.
5. Kim, D. , Doyle, E. K., Wisnowski, J. L., Kim, J. H. and Haldar, J. P. (2017), Diffusion‐relaxation correlation spectroscopic imaging: A multidimensional approach for probing microstructure. Magn. Reson. Med., 78: 2236-2249.
6. Woo J, Stone M, Prince JL. Multimodal registration via mutual information incorporating geometric and spatial context. IEEE Trans Image Process. 2015;24(2):757–769. doi:10.1109/TIP.2014.2387019
7. Henry GH, Malewska A, Joseph DB, et al. A Cellular Anatomy of the Normal Adult Human Prostate and Prostatic Urethra. Cell Rep. 2018;25:3530-3542.e5
8. Lemberskiy G, Fieremans E, Veraart J, Deng FM, Rosenkrantz AB, Novikov DS. Characterization of prostate microstructure using water diffusion and NMR relaxation. Front Phys. 2018;6:91
9. Kwak JT, Sankineni S, Xu S et al. Correlation of magnetic resonance imaging with digital histopathology in prostate. Int J CARS 2016;11(4):657–666.
10. Sabouri, S. , Fazli, L. , Chang, S. D., Savdie, R. , Jones, E. C., Goldenberg, S. L., Black, P. C. and Kozlowski, P. (2017), MR measurement of luminal water in prostate gland: Quantitative correlation between MRI and histology. J. Magn. Reson. Imaging, 46: 861-869
Figures
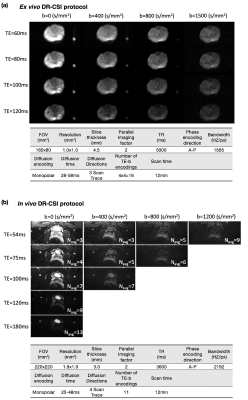
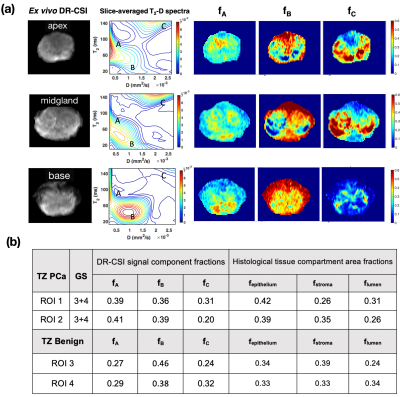
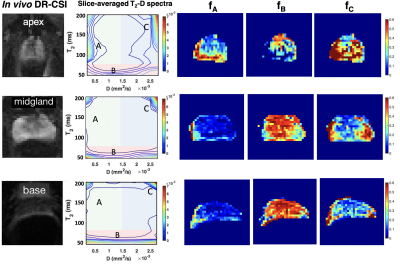
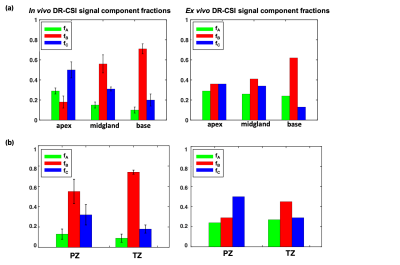
Figure 4. (a) The variations in in vivo (n=4 healthy subjects) and ex vivo (n=1 specimen) DR-CSI signal component fractions values from prostate apex to base. Black bars show the standard deviation across subjects. A general trend of decrease in fA and fC, and increase in fB were observed in healthy subjects, which was consistent with the trend observed in ex vivo DR-CSI. (b) In vivo and ex vivo DR-CSI signal component fraction values in prostate peripheral zone (PZ) and transition zone (TZ). A trend of fC>fA in PZ and TZ, and PZ fC > TZ fC were found for ex vivo and in vivo DR-CSI.