0703
Prospective Validation of an Automated Hybrid Multidimensional MRI Based Tool to Identify Areas for Prostate Cancer Biopsy: Preliminary results1Department of Radiology, University of Chicago, Chicago, IL, United States, 2Philips Research North America, Chicago, IL, United States, 3Department of Urology, University of Chicago, Chicago, IL, United States
Synopsis
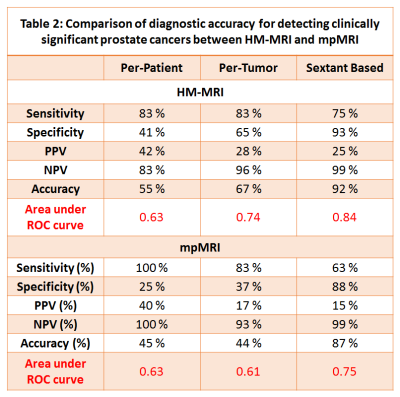
This prospective clinical trial evaluates whether HM-MRI identifies PCa more reliably than random biopsy and/or targets detected based on PI-RADSv2. Patients underwent 3T mpMRI along with HM-MRI. Patients received 12-core TRUS-guided sextant random biopsy. Additional biopsy targets selected by radiologist (≥PI-RADS 3) and suspected PCa based on HM-MRI tissue composition estimates were biopsied, using a Uronav MR-US fusion biopsy device. The diagnostic accuracy of HM-MRI for detecting clinically significant cancers was higher than that of mpMRI on per-tumor (0.74 vs 0.61) and sextant analysis (0.84 vs 0.75). HM-MRI had higher accuracy, sensitivity, specificity and PPV than mpMRI, with similar NPV.
Introduction
Even though mpMRI is increasingly being used for prostate cancer (PCa) diagnosis, around 15-30% of clinically significant cancers are missed even by expert radiologists. In addition, high inter-observer variability in the qualitative interpretation of prostate mpMRI remains a concern (1). Therefore, quantitative and automated tools may improve diagnosis of PCa, leading to better patient outcomes.Prostate tissue composition of gland components: stroma, epithelium, and lumen change with the presence (2) and Gleason grade of PCa (3). Therefore, the distinct MR properties of these tissue components (4) can be exploited to measure tissue composition changes non-invasively using MRI and be used as biomarker for non-invasive PCa detection. A recent feasibility study showed that prostate tissue composition can be measured non-invasively using Hybrid Multidimensional MRI (HM-MRI) and that this approach has the potential to improve PCa diagnosis and determine its aggressiveness (5). Another study validated prostate tissue composition measurement using HM-MRI with reference standard quantitative histology results from whole mount prostatectomy (6).
The goal of this clinical trial is to validate an automated HM-MRI based tool to prospectively identify areas for PCa biopsy. This study evaluates whether HM-MRI based tool identifies PCa more reliably than random biopsy and/or targets detected by an expert radiologist based on PI-RADS v2 in patients undergoing MR-US fusion biopsy.
Materials and Methods
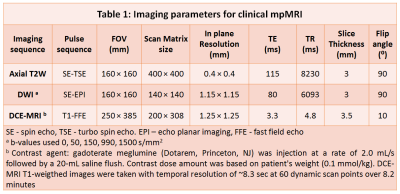
In this prospective clinical trial (ClinicalTrials.gov Identifier: NCT03585660), patients with known (managed by active surveillance) or suspected PCa were recruited with informed written consent. Thirty-four patients (mean age = 64 years, mean PSA = 9.0 ng/ml) underwent MR imaging with a 3T Philips Achieva MR scanner using a 6-channel cardiac phased array coil placed around the pelvis combined with an endorectal coil. mpMRI protocol involved T2W, DWI and DCE-MRI using imaging parameters shown in Table 1. The HM-MRI sequence consisted of a spin-echo module with diffusion sensitizing gradients placed symmetrically about the 180⁰ pulse followed by single shot echo-planar imaging readout. This pulse sequence was used to acquire images with all combinations of TE = 57, 75, 150, 200 ms and b-values = 0, 150, 750, 1500 s/mm2. Tissue composition (fractional volumes of stroma, epithelium and lumen) were calculated by fitting the HM-MRI data to a three compartment signal model, with distinct, paired ADC and T2 values associated with each compartment, similar to the previous studies (5,6).$$ \frac{S}{S_0} =\sum_{n=1}^{n=3} V_n \times exp (-ADC_n \times b - \frac{TE}{T2_n}) $$
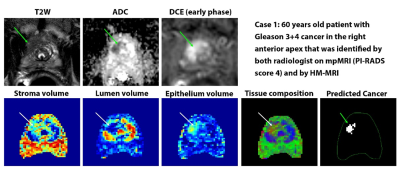
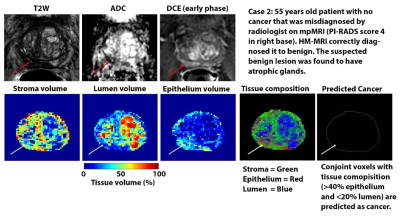
Suspected PCa with elevated epithelium (>40%) and reduced lumen (<20%) meeting the minimum size requirement of 25 mm2 on an axial slice were identified using the HM-MRI tool.
Patients then received 12-core TRUS-guided sextant random biopsy. Additional biopsy targets were selected based on an expert radiologist's interpretation of clinical mpMRI. Lesion with ≥ PI-RADS 3 were biopsied. Up to two additional biopsy targets per patient are selected based on HM-MRI, if different from targets selected by the radiologist, using a Uronav MR-US fusion biopsy device. The biopsy sample underwent H&E staining and histological analysis (cancer diagnosis and Gleason grading) by an expert GU pathologist.
Analyses based on a per-patient, per-tumor and sextant-based analysis were performed. The primary endpoint is area under the ROC curve (AUC), while secondary endpoints are sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy for detecting clinically significant cancers.
Results
The diagnostic accuracy (AUC) of HM-MRI for clinically significant cancers (≥Gleason 3+4), was higher than that of mpMRI on per-tumor (0.74 vs 0.61, p=0.04) and sextant analysis (0.84 vs 0.75, p<0.001). HM-MRI had higher accuracy (67% vs 44%, p<0.05), sensitivity (75% vs 63%, p<0.05), specificity (65 vs 37%, p<0.05) and PPV (28 vs 17%, p<0.05) than mpMRI, with similar NPV (~93-96%). The performance of mpMRI and HM-MRI was similar on a per-patient (AUC = 0.63) basis, possibly due to the small sample size. Table 2 provides detailed statistical endpoints for the comparison of diagnostic accuracy for detecting clinically significant prostate cancers between HM-MRI and mpMRI.Figure 1 and 2 shows representative images from mpMRI (T2W, ADC and early phase DCE-MRI) and HM-MRI.
Discussion
PCa is characterized by increased epithelium and reduced luminal volume compared to normal tissue (2,3,5). Therefore, tissue composition measured by compartmental analysis of HM-MRI data can be used for diagnosing PCa. This study demonstrates that the HM-MRI improves PCa diagnosis compared to mpMRI. Therefore, HM-MRI can be used to guide targeted biopsies and decisions regarding treatment, and has the potential to reduce the number of unnecessary procedures and increase treatment efficiency and efficacy, while increasing sensitivity to clinically significant cancers that might otherwise be missed. This will result in significant benefits for patients while dramatically reducing costs. We are currently improving HM-MRI analysis and extending this study to multiple sites with larger cohorts. We are developing a screening protocol without the use of endorectal coil.Conclusion
The initial results from this study demonstrate that HM-MRI can potentially improve PCa diagnosis by identifying areas of PCa with improved accuracy compared to mpMRI.Acknowledgements
No acknowledgement found.References
1. Niaf E, Lartizien C, Bratan F, Roche L, Rabilloud M, Mège-Lechevallier F, Rouvière O. Prostate Focal Peripheral Zone Lesions: Characterization at Multiparametric MR Imaging—Influence of a Computer-aided Diagnosis System. Radiology 2014;271(3):761-769.
2. Langer DL, van der Kwast TH, Evans AJ, Plotkin A, Trachtenberg J, Wilson BC, Haider MA. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010;255(2):485-494.
3. Chatterjee A, Watson G, Myint E, Sved P, McEntee M, Bourne R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology 2015;277(3):751-762.
4. Bourne RM, Kurniawan N, Cowin G, Stait-Gardner T, Sved P, Watson G, Price WS. Microscopic diffusivity compartmentation in formalin-fixed prostate tissue. Magn Reson Med 2012;68(2):614-620.
5. Chatterjee A, Bourne R, Wang S, Devaraj A, Gallan AJ, Antic T, Karczmar GS, Oto A. Diagnosis of Prostate Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology 2018;287(3):864-872.
6. Chatterjee A, Mercado C, Bourne RM, Yousuf A, Hess B, Antic T, Karczmar G, Oto A. Validation of prostate tissue composition measurement using Hybrid Multidimensional MRI: Correlation with quantitative histology. 2019; Montreal, Canada p0986.
Figures