0699
Metabolic Imaging of a Porcine Model of Acute Lung Injury Using Hyperpolarized [1-13C] Pyruvate MRI1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States, 4Surgery, University of Pennsylvania, Philadelphia, PA, United States, 5Physiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Transpulmonary lactate gradient is strongly correlated with the severity of lung injury and inflammation in ARDS patients. Hyperpolarized [1-13C] pyruvate MRI allows us to quantitatively study altered pyruvate-to-lactate conversion in cancerous and inflamed tissues. We sought to demonstrate the translational potential of this technology for pulmonary metabolic imaging in humans. We performed [1-13C] pyruvate lung MRI in an experimental model of aspiration pneumonitis in pigs, demonstrating this technology’s capacity to detect changes in pulmonary anaerobic metabolism after inflammatory injury in larger species.
Introduction
A large transpulmonary lactate gradient in particular is strongly associated with injury/inflammation severity and poorer outcomes in patients with acute respiratory distress syndrome (ARDS)1–3. The hyper-inflammatory phenotype of ARDS, which presents similar functional and anatomical features on CT to other phenotypes, has twice the mortality rate4. Biomarkers that enable early detection and characterization of pulmonary inflammation may therefore be critical to improving patient outcomes by providing earlier patient stratification and more sensitive monitoring of response to therapy5,6.Hyperpolarized (HP) [1-13C] pyruvate MRI has been used extensively for the quantitative study of altered pyruvate to lactate conversion in tumors and inflamed tissues7–10, and has shown potential for monitoring treatment response in cancer in humans11,12. We previously demonstrated of HP [1-13C] pyruvate MRI’s ability to detect increased pulmonary pyruvate to lactate conversion as a result of inflammatory lung injury in a rat model10. Here, we sought to demonstrate the translational potential of this technology. In light of their similar lung physiology to humans, we performed imaging in a pig model of aspiration pneumonitis in using a setup similar to what would be used clinically. Our findings demonstrated HP [1-13C] MRI’s capacity to detect changes in pulmonary anaerobic metabolism after inflammatory injury.
Materials and Methods
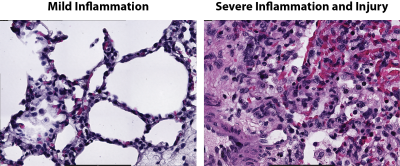
Yorkshire pigs (N=10) were ventilated (VT=10ml/kg, PEEP=0-5 cmH2O, FiO2=0.5-1, f=15 min-1) in supine position. Healthy pigs (N=4) were imaged immediately after preparation. In the remaining pigs (N=6), hydrochloric acid (HCl, pH 1.0) was delivered to the base of the right lung using a bronchoscope, after which animals remained on the ventilator for 7-9 hours. Due to the limited availability of the clinical scanner MRI system for animal use, we did not obtain baseline images in the injured pigs. Animals’ temperature was kept between 36.5-38.5°C during the study using a blanket that circulates heated water. Arterial blood samples were collected from a catheter line placed in the femoral artery for arterial blood gas (ABG) analysis before acid instillation and right before imaging. All imaging was performed using a 3T Siemens Trio clinical MRI system. Localizers and T2-weighted anatomical images were obtained prior to each HP study. 13C images were obtained using a single transmit flex-coil and an 8-channel 13C receive flex-coil. Samples containing 640mg of [1-13C] pyruvate mixed with 15 mM of AH111501 EPA were polarized using a 5T SpinLab hyper-polarizer for 3-5 hours. Samples were melted to yield 30ml of 250mM neutralized isotonic HP [1-13C] pyruvate. 1ml/kg of sample was injected through the ear vein over 30 seconds, and chemical shift imaging was started using a modified FID-CSI sequenced 40 seconds after the start of injection (in-plane FOV=20x20 mm2, slice-thickness=5 cm, matrix-size=16x16, flip-angle=6o, spectral-width=2,560 Hz, TR=52ms). A 15 second breath-hold was applied to minimize imaging artifacts due to respiratory motion. Following euthanasia, lungs were removed, fixed in 10% formalin and stained with hematoxylin and eosin for injury assessment.Results and Discussion
Figure 1 shows pyruvate and lactate maps overlaid on the T2-weighted images in one healthy pig and one injured pig with unilateral injury. The heart was the dominant source of both pyruvate and lactate signals in healthy pigs, and pulmonary pyruvate and lactate signals were localized in the posterior lung due to the more pronounced gravitational gradient of perfusion in larger species. In contrast, pyruvate and lactate signals showed increased signal intensity over the entire lungs in injured animals, but the lactate signal was higher in areas co-localized with severe injury with a signal intensity comparable to that of the heart. Average lactate-to-pyruvate ratio was significantly higher in the injured pigs compared to healthy ones. Post-mortem histological analysis of lung samples showed increased neutrophilic recruitment throughout the entire lung (Figure 2, left) as well as severe inflammation and tissue damage in areas colocalized with hyper-intense regions in the T2-weighted image and higher lactate levels.Conclusions
Our study demonstrates the feasibility of pulmonary metabolic imaging using HP [1-13C] pyruvate MRI in larger species. We consistently observed globally elevated lactate-to-pyruvate ratio in injured animals, with the highest ratios in areas colocalized with severe injury as evident from the anatomical images. These findings are consistent with our earlier study of lung injury in small animals, and suggest that elevated lactate signal in the lungs is a result of recruitment and activation of lung inflammatory infiltrates10. Because pulmonary lactate production significantly increases during inflammatory injury2,13, visualizing the regional distribution of pulmonary lactate may be useful for both early detection and clinical management.Acknowledgements
This work was supported by NIH (Bethesda, MD, USA) grants R01-HL124986 and R01-HL139066.
References
1. De Backer, et al. Am. J. Respir. Crit. Care Med. 156, 1099–1104 (1997).
2. Iscra, F., Gullo, A. & Biolo, G. Bench-to-bedside review: Lactate and the lung. Crit Care 6, 327–329 (2002).
3. Routsi, C. et al. Crit. Care (1999).
4. Thompson, et al. Mass Med. Soc 377, 562–572 (2017).
5. Calfee, C. S. et al. Am. J. Respir. Crit. Care Ldots (2016).
6. Calfee, C. S. et al. Lancet Respir. Med. (2018) doi:10.1016/S2213-2600(18)30177-2.
7. Siddiqui, S. et al. Adv. Drug Deliv. Rev. (2016) doi:10.1016/j.addr.2016.08.011.
8. Keshari, K. R. et al. Chem. Soc. Rev. (2014).
9. Cunningham, C. H. et al. Circ. Res. CIRCRESAHA.116.309769 (2016) doi:10.1161/CIRCRESAHA.116.309769.
10. Pourfathi, M., et al. nature.com 8, 280 (2018).
11. Kurhanewicz, J. et al. NeoPlasia 21, 1–16 (2019).
12. Aggarwal, et al. Eur. Urol. (2017) doi:10.1016/j.eururo.2017.07.022.
13. Wellman, et al. Anesthesiology 125, 992–1004 (2016).
Figures