0697
Imaging Acute Metabolic Changes in Mild Traumatic Brain Injury Patients using Hyperpolarized [1-13C]Pyruvate1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3GE Healthcare, GE Healthcare, Dallas, TX, United States, 4Physical Medicine and Rehabilitation, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Neurosurgery, University of Texas Southwestern Medical Center, Dallas, TX, United States, 6Electrical Engineering, University of Texas Dallas, Richardson, TX, United States
Synopsis
A major challenge of treating traumatic brain injury (TBI) patients is the simultaneously occurring complex secondary injury processes following the primary injury. The secondary events such as cerebral hyperglycolysis and mitochondrial failure develop over minutes to months after the primary injury. This case report details the first time hyperpolarized [1-13C]pyruvate imaging in TBI patients to examine regional metabolic changes in the brain post-traumatic injury. We observed an increased conservation of pyruvate to lactate at the injured sites as well as reduced bicarbonate production.
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in the United States, contributing to approximately 30% of all injury-related deaths [1]. A major challenge of TBI patients is the simultaneously occurring complex secondary injury processes following the primary injury. After the initial loss of brain cells due to the primary damage, the surviving tissue will undergo metabolic shifts to compensate for the immediate loss, resulting in the development of potentially hazardous secondary metabolites and further damage. The secondary events such as cerebral hyperglycolysis and mitochondrial failure develop over minutes to months after the primary injury, providing a potential window of opportunity for therapeutic intervention. Given early, this intervention may prevent or reduce secondary brain damage, directly impacting long-term patient outcome. Therefore, the noninvasive detection and characterization of pathophysiology in TBI patients during the acute and early sub-acute stages, will have critical clinical implications for the early diagnosis of individuals with the highest risk of poor neurological outcomes and will be vital for identifying and developing effective therapies. Previous studies using hyperpolarized [1-13C]pyruvate demonstrated that increased [1-13C]lactate production in the injured brain tissue due to the microglial activation [2,3]. Recent longitudinal animal studies using [1-13C]pyruvate for monitoring alterations of energy metabolism demonstrated oxidative phosphorylation shifts (decreased [13C]HCO3–) to increase in undamaged tissue peaks during the acute stage of TBI [4]. In this study, we translates the animal findings to observe the metabolic changes in the human brain after acute imaging using hyperpolarized [1-13C]pyruvate.Methods
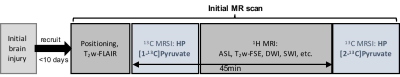
All the studies were performed using a clinical SPINlab polarizer (GE Healthcare), a 3T MR scanner (GE Healthcare, 750w Discovery), and a 13C/1H dual-frequency RF head coil (Clinical MR Solutions) [5]. The patient was a 35-year old African American male with a 2-cm lactation to the left side of the forehead due to blunt force trauma (whipped by a metal gun) with a Glasgow coma scale (GCS) score of 15 and no loss of consciousness. After being brought to Parkland Hospital Emergency Department, a UT Southwestern-affiliated hospital in Dallas, a full workup was initiated and CT scans showed no evidence of any further internal damage. The patient was released with minor laceration care and brought in for research testing the following day. The subject was imaged with a brain MR protocol, which includes two injections of hyperpolarized [1-13C]pyruvate (IND#: 133229) with at least a 45-min interval between the injections. The first hyperpolarized pyruvate solution was injected after a two-dimensional T2-weighted FLAIR scan. For each 13C acquisition, a volume of 250-mM hyperpolarized pyruvate corresponding to a 0.1 mmol/kg dose was injected, followed by a 25-mL saline flush. A single-slice 2D dynamic spiral chemical shift imaging (spiral CSI; FOV = 24×24 cm2, matrix size = 16×16, slice thickness = 3 cm, variable flip angle up to 30o per timepoint, TR = 5 sec, 7 spatial interleaves in spiral readout, spectral width = 814 Hz, 48 echoes) was used for dynamic imaging of hyperpolarized 13C signals [6]. The remaining 1H images were acquired during the 45-min time interval. Finally, the dynamic 13C spiral CSI was repeated with another injection of hyperpolarized [1-13C]pyruvate. The imaging protocol was approved by the local Institutional Review Board, and summarized in Figure 1.Results and Discussion
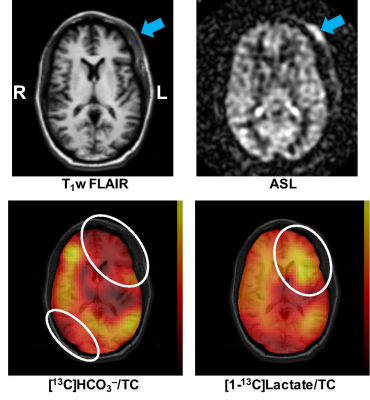
Figure 2 shows axial images of the acute TBI patient 28 hours after the initial injury. Although there was no anatomical brain damage, significantly decreased [13C]HCO3– and increased [1-13C]lactate production was measured in the time-averaged spiral CSI images. These pilot studies demonstrate potential clinical values of hyperpolarized pyruvate for imaging TBI patients and our ability to perform the proposed translational study. Interestingly, significantly reduced HCO3– production was observed in the right posterior brain region, suggesting possible coup contrecoup injury in the region. The 13C images are masked and overlaid on top of 1H image. Longitudinal monitoring with a larger patient population will be needed to further evaluate the utility of hyperpolarized pyruvate for noninvasive assessment of the TBI metabolism.Conclusion
TBI patients were imaged using hyperpolarized [1-13C]pyruvate for the first time. The mildly injured brain region showed increased lactate and decreased HCO3– production despite no anatomical damage in the brain, demonstrating the sensitivity of hyperpolarized pyruvate to altered metabolism in TBI.Acknowledgements
Personnel Support: We appreciate the clinical research team of the Advanced Imaging Research Center at UT Southwestern – Craig Malloy, Jeannie Baxter, Kelley Durner, Lucy Christie, Maida Tai and Salvador Pena.
Funding Support: The Texas Institute of Brain Injury and Repair; The Mobility Foundation; National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award.
References
1. C. A. Taylor, J. M. Bell, M. J. Breiding, and L. Xu. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66, 1–16 (2017).
2. S. J. DeVience, X. Lu, J. Proctor, P. Rangghran, E. R. Melhem, R. Gullapalli, G. M. Fiskum, and D. Mayer, “Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-(13)C]pyruvate.,” Sci Rep, vol. 7, no. 1, p. 1907 (2017).
3. C. Guglielmetti, A. Chou, K. Krukowski, C. Najac, X. Feng, L.-K. Riparip, S. Rosi, and M. M. Chaumeil, “In vivo metabolic imaging of Traumatic Brain Injury.,” Sci Rep, vol. 7, no. 1, p. 17525 (2017).
4. E. Hackett, J. Chen, L. Ingle, B. Bartnik-Olson, and J. M. Park. Longitudinal changes in pyruvate metabolism in the brain after traumatic injury. The 3rd Joint Symposium of the International and National Neurotrauma Societies and AANS/CNS Section on Neurotrauma and Critical Care, Toronto, Canada. #657 (2018).
5. J. Ma, R. Hashoian, C. Sun, S. Wright, A. Ivanishev, R. Lenkinski, C. Malloy, A. Chen, and J. M. Park, “Development of 1H/13C RF head coil for hyperpolarized 13C imaging of human brain.,” ISMRM. #568 (2019).
6. J. M. Park, J. Liticker, C. Harrison, G. Reed, T. Hever, J. Ma, R. Martin, D. Mayer, R. Hashoian, C. Madden, M. Pinho, and C. Malloy, “Feasibility and reproducibility of imaging brain metabolism using hyperpolarized 13C pyruvate in humans.,” ISMRM. #4311 (2019).