0696
Providing a clinical pipeline for using the sodium-23 resonance to calibrate for in vivo hyperpolarized carbon-13 experiments.1Unviesity of Cambridge, Cambridge, United Kingdom, 2University of Birmingham, Birmingham, United Kingdom, 3Aarhus University, Aarhus, Denmark, 4Technical University of Denmark, Copenhagen, Denmark, 5Cancer Research UK, Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 6University of Cambridge, Cambridge, United Kingdom, 7GE Healthcare, Munich, Germany
Synopsis
Hyperpolarized 13C MRI is an emerging clinical technique to probe metabolism. Calibration of transmit gain and centre frequency is challenging, due to the low endogenous 13C signal. Pre-scan is typically performed by adding an external phantom for reference, however this is challenged by the shim volume inside the subject and the RF coil excitation and receptions profiles. We demonstrate the ability to use the sodium-23 resonance to accurately prescan prior to 13C experiments, using single tuned 13C coils in a 3T MRI system. This provides an important workflow improvement for the adoption of hyperpolarized 13C imaging into clinical practise.
Introduction
Hyperpolarized carbon-13 MRI is a powerful, clinical technique to probe in vivo metabolism, and recent studies have demonstrated the technique in the brain, heart, and in cancer (1–4) The calibration of the system centre frequency and radiofrequency power is commonly achieved using external phantoms but errors in this calibration will significantly affect data quality. Previous work has assessed the use of dual tuned 23Na/13C coils to calibrate 13C experiments as part of the prescan (5,6) and here we develop this idea by using dedicated commercially available 13C coils to provide this data. Here we show that the sodium-23 resonance can be reliably used to calibrate clinical systems prior to data acquisition using dedicated, clinically available, 13C tuned coils providing all the necessary system parameters for successful data acquisition.Methods
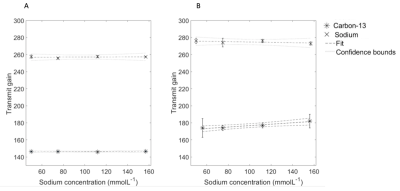
System centre frequency and transmit gain (TG) estimationFour cylindrical phantoms filled with saline (150-17 mMoL-1 NaCl) with a urea phantom (8 MoL-1) on top were placed in the scanner (3T, HDx, GE Healthcare, WI) with either two 13C tuned 8-channel paddle coils or a single loop receive coil placed either side of the phantom or on top of the phantom, respectively. A Helmholtz loop transmitter (clamshell) was used for B1 transmission. The 13C urea and 23Na centre frequency and TG were acquired using a pulse-acquire Bloch-Siegert acquisition (hard pulse, pulse width = 0.5 ms, repetition time (TR) = 2 s, echo time (TE) = 0.5 ms, flip angle (FA) = 90 degrees)(7). The ratiometric difference in centre frequency between 23Na/1H and 13C-urea were averaged over all experiments. The difference in TG (dB) between 23Na and 13C was calculated for all experiments. A linear fit between the TG at different loading states per nuclei was calculated.
In vivo experiments
Six female Danish land pigs were placed supine in the scanner. The single loop coil was placed on the biceps femoris muscle of the right lower limb, and used for acquisition with a 13C-lactate (4MolL-1) phantom placed in the FOV of the coil. The 23Na and 13C centre frequency were recorded, as well as the TG required for 23Na and 13C as described above before data acquisition (partially self-refocusing since pulse, spectral bandwidth = 5 kHz, number of samples = 2048, TR = 1 s, 128 time points, FA = 12 degrees, slice thickness = 40 mm). Spectral data were post-processed using matching pursuit fitting and kinetic modelling as well as calculating ratiometric results (lactate:pyruvate, bicarbonate:pyruvate) (8). Kinetic and ratiometric results were averaged over all experiments, and a linear correlation between kPL and lactate:pyruvate was performed. All processing and statistical analysis was performed in Matlab (2018a, The Mathworks, MA).
Coil profile estimation
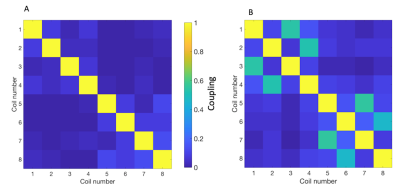
The RF amplifier was disabled and a noise only acquisition performed with the 8-channel paddle coils to calculate the noise covariance matrix at both 13C and 23Na frequencies.
Transmit B1 Simulations
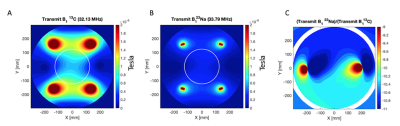
Simulations of the clamshell B1+ performance at both 13C and 23Na frequencies was performed (CST, Darmstad, Germany) and the ratio between 13C and 23Na B1+ fields (T) computed.
Results
System f0 and TG calibration can be performed with the 23Na resonance for 13C experimentsThe ratiometric difference in 23Na and 13C Urea f0 at 3T was 1.05180 ± 0.00001, with the mean difference in TG for 23Na and 13C was 10.4 +/- 0.6 dB. Simulated B1+ profiles agreed with this (Figure 2), with a predicted difference in 10.4 +/- 0.2 dB between 23Na and 13C. There was no significant correlation between saline loading and TG (R2 < 0.1, p > 0.05). Simulations showing the transmit B1 field at the 13C and 23Na frequencies can be found in Figure 2A and B, respectively; Figure 2C shows the ratiometic difference between the two.
13C coil element noise coupling increases at 23Na frequency
Noise correlation between the 8 channels in the paddle coil revealed good linearity at 13C frequency, however increased off-diagonal correlation was observed at 23Na f0, shown in Figure 3A and B, respectively.
In vivo hyperpoalrized 13C experiments are successful using a 23Na prescan
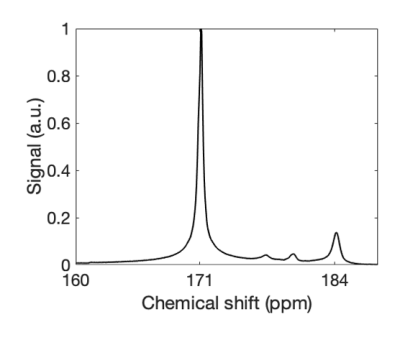
Using the 23Na prescan, hyperpolarized 13C MRS was successfully performed from the musculature of six pigs. The frequency scaling constant between 23Na and [1-13C] pyruvate was 1.05179 ± 0.00001. Kinetic analysis revealed a mean kPL of 0.007 +/- 0.002 s-1 and correlated with lactate:pyruvate ratio (R2 = 0.9, p = 0.01). Example summed spectra are seen in Figure 4.
Discussion
This study has provided the translational step required to provide a clinical pipeline for successful, accurate, prescan measures for hyperpolarized 13C experiments using the 23Na resonance. Due to the high natural abundance of 23Na in vivo, combined with the low frequency difference at 3T, it is possible to utilise this technique with dedicated, commercially available, coils – providing the translation step required for clinical application and uptake of this technique.Acknowledgements
This study was funded by the Lundbeck Foundation, Medical Research Council, Cancer Research UK, and the Little Princess Trust.References
1. Zaccagna F, Grist JT, Deen SS, et al. Hyperpolarized carbon-13 magnetic resonance spectroscopic imaging: a clinical tool for studying tumour metabolism. Br. J. Radiol. 2018;91:20170688.
2. Cunningham CH, Lau JYC, Chen AP, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. 2016;119:1177–1182.
3. Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res 2018.
4. Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn. Reson. Med. 2018;00.
5. Hancu I, Watkins R, Kohler SJ, Mallozzi RP. Accurate flip-angle calibration for 13C MRI. Magn. Reson. Med. 2007;58:128–133.
6. Hancu I, Wood SJ, Piel J, et al. Three-frequency RF coil designed for optimized imaging of hyperpolarized, 13C-labeled compounds. Magn. Reson. Med. 2008;60:928–933.
7. Schulte RF, Sacolick L, Deppe MH, et al. Transmit gain calibration for nonproton MR using the Bloch-Siegert shift. NMR Biomed. 2011;24:1068–1072.
8. Khegai O, Schulte RF, Janich M a, et al. Apparent rate constant mapping using hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 2014;27:1256–65.
Figures