0693
Transportable hyperpolarized glucose samples: towards remote dissolution DNP1Health Technology, Danish Technical University, Kongens Lyngby, Denmark, 2IPHYS, EPFL, Lausanne, Switzerland
Synopsis
Our vision is to enable delivery of hyperpolarized compounds to MR-facilities that currently have no access to hyperpolarization technology. Today this is not the case and represents a main shortcoming of hyperpolarized-MR via dissolution Dynamic Nuclear Polarization (dDNP). The cause is the presence, in the dDNP sample, of organic free radicals necessary to generate the hyperpolarization. We herein present a paradigm shift in the technique built on the employment of photo-induced thermally-labile free radicals. We demonstrate quenching of the paramagnetic species while preserving most of the polarization in the case of hyperpolarized glucose.
Introduction
13C Hyperpolarized Magnetic Resonance (HP-MR) via dissolution Dynamic Nuclear Polarization (dDNP) has the potential of revolutionizing diagnostic radiology.1-3 However, the type of applications and the diffusion of this technique on a wider scale is limited by the short life-time of the HP state after dissolution. Therefore, HP samples have to be prepared as close as possible to the MR apparatus employing an expensive, technically demanding machine with high running costs (i.e. the dDNP polarizer), making unlikely to equip each MR facility with hyperpolarization. The culprits are the unpaired electron spins present in the DNP sample in form of organic free radicals. They are needed for the DNP process, but they also prevent extraction of the HP sample in the solid state for the sake of storing, transporting and eventually dissolving the sample far away from the production site. We herein propose a paradigm shift in the technique: to make hyperpolarization transportable. The idea behind builds on a recent innovation in the field of dDNP where photo-induced thermally-labile free radicals are employed.4-6 As the paramagnetic molecules decompose (quench) at around 190K it is possible to eliminate them already in the solid state inside the polarizer while retaining the DNP.7 In this work, we present our progress in the technique. We focus on the translation from proof-of-principle experiment to user friendly technique. For this purpose we chose HP glucose as test substrate, and deuterated trimethylpyruvic acid (d9-TriPA) as UV-radical precursor.5Methods
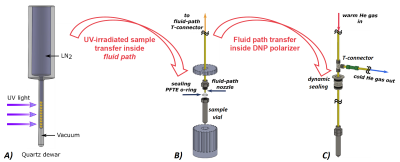
[[U-13C6,1,2,3,4,5,6,6-d7]-D-glucose was dissolved in 100uL of glycerol:water 1:1 (v/v) to obtain a final glucose concentration of 2.2M; d9-TriPA was finally added in amount corresponding to 10% of the final volume. Ten droplets of 4.0±0.5µL of solution were poured in liquid nitrogen to form frozen beads. The latter were UV-irradiated at 77K for 300s with a high power (20W/cm2) broad-band UV source (Dymax BlueWave75) to generate the radicals (see Figure1A). After irradiation the beads, kept in liquid nitrogen, were transferred to a custom designed fluid path (CFP) to be loaded inside a homebuilt dDNP polarizer working at 1.10±0.05K and 6.7T (see Figure1B). Microwaves were delivered from a solid-state source (Polarize, DK). The sample was polarized at best DNP conditions (60mW of output power at 188.19GHz; 20MHz of frequency amplitude modulation at a rate of 1kHz). The 13C solid-state NMR signal was monitored using a low-temperature probe connected to a benchtop spectrometer (Magritek, NZ). After achieving maximum polarization, microwaves were switched OFF, the sample lifted outside the liquid He bath, warmed up above the radical quenching point by blowing room temperature He gas inside the CFP (see Figure1C), and finally moved back inside the NMR coil for measurements.Results and Discussion
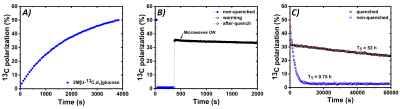
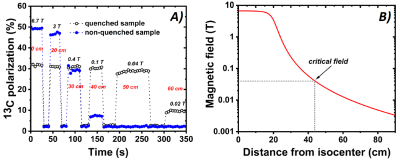
UV-light irradiation generated in the solid sample a radical concentration as high as 40±2mM. This sample formulation allowed us to reach a glucose 13C polarization of 50±5% in approx. 1h (See Figure2A). Complete quenching of the radical was achieved by blowing He at 3bar for 5s. The design of the CFP was fundamental for this purpose. The CFP was suitable for cold loading of solid samples and reusable thanks to the threaded leak-tight connection of the sample vial. Moreover, it allowed to warm the sample above the radical quenching temperature, while keeping the polarizer at low pressure. This had two consequence: ease of operations and efficient heat exchange between the flowing gas and the frozen sample. Figure2B shows the results of thermalization: approximately 2/3 of the polarization achieved in the solid state could be persevered and the absence of radical was confirmed by the observation of no DNP build-up after switching back ON the microwaves. Removing the radicals from the HP sample had a dramatic influence on the 13C spin-lattice relaxation time: its value, measured at 6.7T and 4K, increased from 0.75h to 53h (see Figure2C). A fundamental point, in vision of extracting the HP solid sample for storage and transport, was to investigate its behavior as a function of magnetic field and temperature variations, in particular while moving it to positions with increasing distances from the isocenter of the polarizer magnet. To this regard we report, in Figure3A, the results for a thermalized (thus radical free) sample and a non-thermalized sample. It is clear that the presence of radicals dramatically affected the polarization already when exposing the sample to a magnetic field of 400mT, corresponding to a distance from the isocenter of 30 cm (refer to the polarizer magnetic field simulation, Figure 3B). Nevertheless, also the thermalized sample experienced a polarization drop when exposed to a magnetic field smaller than 40mT.Conclusion and Perspectives
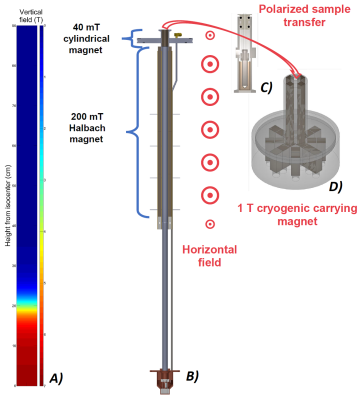
Our vision is to enable delivery of hyperpolarized compounds to universities and hospitals that currently have no access to hyperpolarization technology and provide researchers and medical doctors with a simple setup (CFP plus dissolution station) to perform dissolution “off-site”, somehow similar to what is currently done in nuclear medicine for the distribution of radiopharmaceuticals. We are currently working on a new design of the polarizer that employs permanent magnets to guarantee a magnetic field sufficiently high to extract the HP, radical free and long relaxing sample (see Figure 4), while preserving most of the polarization.Acknowledgements
The research leading to these results has received funding from the Danish National Research Foundation (DNRF124) and the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 713683 (COFUNDfellowsDTU).References
1. Ardenkjaer-Larsen, J. H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M. H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. P Natl Acad Sci USA 2003, 100 (18), 10158-10163.
2. Kurhanewicz, J.; Vigneron, D. B.; Ardenkjaer-Larsen, J. H.; Bankson, J. A.; Brindle, K.; Cunningham, C. H.; Gallagher, F. A.; Keshari, K. R.; Kjaer, A.; Laustsen, C.; Mankoff, D. A.; Merritt, M. E.; Nelson, S. J.; Pauly, J. M.; Lee, P.; Ronen, S.; Tyler, D. J.; Rajan, S. S.; Spielman, D. M.; Wald, L.; Zhang, X.; Malloy, C. R.; Rizi, R. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21 (1), 1-16.
3. Nelson, S. J.; Kurhanewicz, J.; Vigneron, D. B.; Larson, P. E. Z.; Harzstark, A. L.; Ferrone, M.; van Criekinge, M.; Chang, J. W.; Bok, R.; Park, I.; Reed, G.; Carvajal, L.; Small, E. J.; Munster, P.; Weinberg, V. K.; Ardenkjaer-Larsen, J. H.; Chen, A. P.; Hurd, R. E.; Odegardstuen, L. I.; Robb, F. J.; Tropp, J.; Murray, J. A. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-C-13]Pyruvate. Sci Transl Med 2013, 5 (198), 198ra108 1-10.
4. Capozzi, A.; Karlsson, M.; Petersen, J. R.; Lerche, M. H.; Ardenkjaer-Larsen, J. H. Liquid-State C-13 Polarization of 30% through Photoinduced Nonpersistent Radicals. J Phys Chem C 2018, 122 (13), 7432-7443.
5. Capozzi, A.; Patel, S.; Gunnarsson, C. P.; Marco-Rius, I.; Comment, A.; Karlsson, M.; Lerche, M. H.; Ouari, O.; Ardenkjaer-Larsen, J. H. Efficient Hyperpolarization of U-(13) C-Glucose Using Narrow-Line UV-Generated Labile Free Radicals. Angewandte Chemie 2019, 58 (5), 1334-1339.
6. Eichhorn, T. R.; Takado, Y.; Salameh, N.; Capozzi, A.; Cheng, T.; Hyacinthe, J. N.; Mishkovsky, M.; Roussel, C.; Comment, A. Hyperpolarization without persistent radicals for in vivo real-time metabolic imaging. P Natl Acad Sci USA 2013, 110 (45), 18064-18069.
7. Capozzi, A.; Cheng, T.; Boero, G.; Roussel, C.; Comment, A. Thermal annihilation of photo-induced radicals following dynamic nuclear polarization to produce transportable frozen hyperpolarized 13C-substrates. Nat Commun 2017, 8, 15757.
Figures