0691
Hyperpolarized [1-13C] Glycerate as Probe to Assess Glycolytic Activity in a Rat Model of Hepatocellular Carcinoma
Jun Chen1, Evan LaGue2, Junjie Li1, Edward Hackett1, Ian Corbin1, Kelvin Billingsley2, and Jae Mo Park3,4
1AIRC, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2Chemistry and Biochemistry, California State University Fullerton, Fullerton, CA, United States, 3UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 4Electrical Engineering, University of Texas at Dallas, Richardson, TX, United States
1AIRC, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2Chemistry and Biochemistry, California State University Fullerton, Fullerton, CA, United States, 3UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 4Electrical Engineering, University of Texas at Dallas, Richardson, TX, United States
Synopsis
Hyperpolarized [1-13C] glycerate was used to study the in vivo glycolytic activity in a rat model of hepatocellular carcinoma (HCC). Carbon-13 labeled glycolytic intermediate phosphorenolpyruvate (PEP) was detected in the tumor in addition to pyruvate and lactate peaks. The in vivo results were confirmed by high resolution 13C NMR spectra of tissue extracts, after steady-state infusion of [2,3-13C2] glycerate. The results illustrate the potential of [1-13C] glycerate as a metabolic probe for assessing glycolytic flux.
Introduction
Most cancer cells can rewire their metabolism to increase glucose uptake with a preference for glycolysis and fermentation, which is known as the Warburg effect (1). The pyruvate kinase (PK) catalyzes the last step of glycolysis which converts PEP to pyruvate and contributes to anabolic metabolism. In particular, the M2 isoform of PK, PKM2, promotes tumorigenesis by regulating Warburg effect in many cancers including HCC (2, 3). While hyperpolarized 13C-pyruvate has shown its utility to detect the Warburg effect via increased 13C-lactate production, the assessment is indirect as it detects upregulated lactate dehydrogenase (LDH) activity. Recently, we developed [1-13C] glycerate as a new hyperpolarization probe that directly assesses in vivo glycolysis and demonstrated feasibility in fed and fasted rat livers (4). Glycerate can be converted into glycolytic intermediates easily to trace the metabolic flux of glycolysis. In this study, we applied hyperpolarized [1-13C] glycerate to a rat hepatoma model to demonstrate its capability to detect Warburg metabolism and altered PK activity in cancer. The detailed utilization of glycerate in the liver and the tumor was further confirmed by 13C NMR isotopomer analysis of ex vivo tissue samples after steady-state infusion of [2,3-13C2] glycerate.Methods
13C-labeled glycerate was synthesized as described in [4]. For dynamic nuclear polarization (DNP), 3.2 M of [1-13C] glycerate was prepared in 3:2 w/w water:glycerol with 15-mM OX063 and polarized using a GE SPINlab. Healthy male Wistar rats were given 100 mg/kg diethylnitrosamine (DEN) once a week intraperitoneally for 5 weeks (5), then fed with 0.01% DEN water for 8 weeks. Tumors appeared after 13-16 weeks. In vivo MR spectroscopy (MRS) experiments were performed at a clinical 3T MR scanner (GE Discovery 750w). A custom-built 13C surface coil (single loop, Ø = 28 mm) was placed on top of the liver area for both radiofrequency (RF) excitation and data acquisition. 80-mM hyperpolarized [1-13C] glycerate was injected intravenously as a bolus (1 mmol/kg body weight, up to 4.0 mL, injection rate = 0.25 mL/s), immediately followed by a dynamic 13C pulse-and-acquire scan (10o hard pulse excitation, repetition time = 3 sec, scan time = 4 min). Natural abundance lipid peaks were removed by subtracting baseline spectrum from glycerate injected spectra (6). For comparison, hyperpolarized [1-13C] pyruvate was prepared for a single time-point free-induction decay chemical shift imaging (FID CSI; FOV = 80x80 mm2, matrix = 16x16). To validate the in vivo glycerate results, bolus injection of 0.25mg/g [2,3-13C2] glycerate was given to the rat through tail vein followed by a two-hour infusion of 0.006 mg/g body weight [2,3-13C2] glycerate at 4.8 mL/hr. Once the steady-state condition was achieved, tumor tissue (or normal liver) was harvested, then immediately freeze-clamped with liquid N2. The frozen tissue was extracted with percholoric acid and the 13C NMR spectra were acquired using a 600 MHz Oxford NMR system with a cryogenically-cooled 13C probe for isotopomer analysis.Results and Discussion
All the DEN-treated rats developed tumors. Healthy rat liver produced [1-13C] lactate at 185.2 ppm and pyruvate at 172.1 ppm as well as bicarbonate at 161.4 ppm from hyperpolarized [1-13C] glycerate at 178.9 ppm (Fig.1A). Additional peak appeared in the time-averaged spectra from DEN rats at 173.8 ppm, which is aligned with [1-13C] PEP resonance (Fig.1B) (7), whereas no bicarbonate peak was detected. The lacate to pyruvate ratio decreased dramatically, which might be related changes in NAD+/NADH ratio in HCC (8). The PEP signal was peaked at 12-15 sec from the start of hyperpolarized glycerate injection (Fig.2), which was earlier than the other products (15-18 sec for lactate, 18-21 sec for pyruvate). The pyruvate to lactate ratio was also significantly increased in the tumor as compared to normal liver, which might be related to the change in redox state in cancer. The appearance of PEP implies that glycolysis is elevated with PKM2 activity skewed for biosynthesis (9). The increased pyruvate is likely from upregulated PEP phosphorylation of phosphoglycerate mutase (PGAM1) (10). The in vivo results were consistent with the steady-state 13C NMR spectra, acquired from the freeze-clamped tissues: increased glycerate flux to alanine and lactate, in addition to an extra peak at [3-13C] pyruvate resonance (Fig.3). The CSI with hyperpolarized [1-13C] pyruvate indicates that injected pyruvate was delivered rapidly to the tumor and the tumor is highly glycolytic, producing increased lactate in the tumor (Fig.4). However, alanine production was decreased in the tumor unlike other hepatoma models such as Morris hepatoma (11).Conclusion
In this work, we demonstrated that [1-13C] glycerate can provide assessment of glycolytic activity of rat HCC in vivo via hyperpolarized 13C MRS. We were able to detect the metabolites from the last steps of glycolysis, [1-13C] PEP and [1-13C] pyruvate, in addition to [1-13C] lactate. The [1-13C] lactate to [1-13C] pyruvate level provide opportunities for evaluating intracellular redox states in biochemical investigations [1-13C] Alanine could not be resolved from the large [1-13C] glycerate peak. Hyperpolarized [1-13C] glycerate and [1-13C] pyruvate, therefore, can be complementary tools for comprehensively assessing Warburg effect by measuring associated enzyme activities in vivo: PK, PDH, LDH, ALT, and PDH. Future study will focus on using and improving [1-13C] glycerate to trace the metabolic flux change during tumorigenesis and treatment.Acknowledgements
The Texas Institute of Brain Injury and Repair; The Mobility Foundation; National Institutes of Health of the United States (SC1 GM127213, R01 CA215702, P41 EB015908, S10 OD018468); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award.References
1. Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8(6):519-30. 2. Imamura K, Tanaka T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J Biochem. 1972;71(6):1043-51. 3. Schneider J, Neu K, Grimm H, Velcovsky HG, Weisse G, Eigenbrodt E. Tumor M2-pyruvate kinase in lung cancer patients: immunohistochemical detection and disease monitoring. Anticancer Res. 2002;22(1a):311-8. 4. Park JM, Wu M, Datta K, Liu SC, Castillo A, Lough H, et al. Hyperpolarized Sodium [1-(13)C]-Glycerate as a Probe for Assessing Glycolysis In Vivo. J Am Chem Soc. 2017;139(19):6629-34. 5. Ding YF, Wu ZH, Wei YJ, Shu L, Peng YR. Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine. J Cancer Res Clin Oncol. 2017;143(5):821-34. 6. Hu S, Zhu M, Yoshihara HA, Wilson DM, Keshari KR, Shin P, et al. In vivo measurement of normal rat intracellular pyruvate and lactate levels after injection of hyperpolarized [1-(13)C]alanine. Magn Reson Imaging. 2011;29(8):1035-40. 7. Timm KN, Hartl J, Keller MA, Hu DE, Kettunen MI, Rodrigues TB, et al. Hyperpolarized [U-(2) H, U-(13) C]Glucose reports on glycolytic and pentose phosphate pathway activity in EL4 tumors and glycolytic activity in yeast cells. Magnetic resonance in medicine. 2015;74(6):1543-7. 8. Bahitham W, Liao X, Peng F, Bamforth F, Chan A, Mason A, et al. Mitochondriome and cholangiocellular carcinoma. PLoS One. 2014;9(8):e104694. 9. Gui DY, Lewis CA, Vander Heiden MG. Allosteric regulation of PKM2 allows cellular adaptation to different physiological states. Sci Signal. 2013;6(263):pe7. 10. Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329(5998):1492-9. 11. Darpolor MM, Yen YF, Chua MS, Xing L, Clarke-Katzenberg RH, Shi W, et al. In vivo MRSI of hyperpolarized [1-(13)C]pyruvate metabolism in rat hepatocellular carcinoma. NMR Biomed. 2011;24(5):506-13.Figures
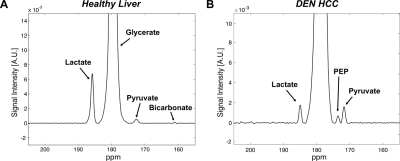
Time-averaged 13C
spectra from the liver of (A) control and (B) DEN rats after a bolus injection
of hyperpolarized 80-mM [1-13C] glycerate.
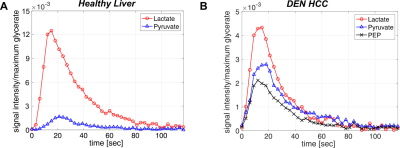
Dynamic changes of products from hyperpolarized [1-13C] glycerate. (A) Normal and (B) DEN rats
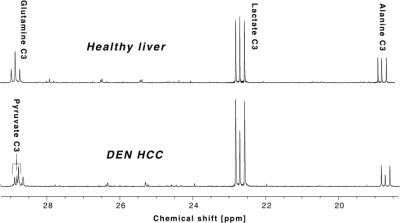
13C NMR spectra from freeze-clamped tissue
samples. (Top) Normal liver from healthy control and (Bottom)
HCC from DEN rat
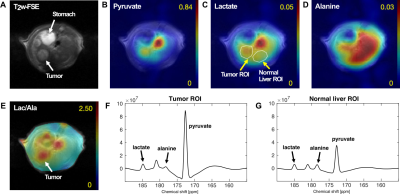
CSI of hyperpolarized [1-13C] pyruvate and the
products in DEN rat with hepatoma.
(A) T2-weighted 1H MRI. Metabolite maps of (B)
hyperpolarized [1-13C]
pyruvate, (C) [1-13C] lactate and (D) [1-13C] alanine,
overlaid on the corresponding 1H MRI. (E) Lactate-to-alanine
ratio map. Spectra averaged over (F) tumor and (G) normal-appearing liver
regions of interest (ROI), shown in (C).