0689
Metabolism of the hyperpolarized neuroprotective agents [1-13C] lactate and [1-13C] pyruvate in a mouse model of transient ischemic stroke1Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 2Laboratory of Functional and Metabolic Imaging, École polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Clinical Neurosciences, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 4Center for Biomedical Imaging - Animal Imaging and Technology (CIBM-AIT), École polytechnique fédérale de Lausanne (EPFL), Lausanne, Switzerland, 5Image Guided Intervention Laboratory, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
Stroke is a major cause of death and disability. Neuroprotective strategies could ameliorate patient recovery. Pyruvate and lactate were found neuroprotectant in preclinical studies of stroke models. Hyperpolarized 13C MRI provides a new way for real-time molecular imaging. In this work, we hyperpolarize those neuroprotective agents to study changes of their metabolism when administered at their therapeutic dose after ischemic stroke. We found that the metabolism of hyperpolarized lactate is significantly altered after transient cerebral ischemia, whereas moderate changes were depicted with hyperpolarized pyruvate. Those imply that hyperpolarized lactate would potentially be a better theranostic biosensor for stroke.
Introduction
Stroke is a major cause of death and disability worldwide1. 85% of strokes are ischemic and can be treated by restoring the blood flow through thrombolysis or thrombectomy within a narrow time window of 4.5h and 7.3h after ischemic onset2–4.Neuroprotective strategies in acute ischemic stroke could reduce neuronal death and improve patient recovery5. While endogenous lactate increases dramatically after ischemia6, exogenous administration of lactate after ischemic stroke was found neuroprotective in preclinical studies7–9. Similarly, recent in-vitro7 and in-vivo10 studies indicted on neuroprotective properties of pyruvate.
MR of hyperpolarized (HP) endogenous compounds by dissolution dynamic nuclear polarization (dDNP)11 allows monitoring real-time metabolic transformations. [1-13C]pyruvate is the gold standard HP contrast agent12, as the interconversion between HP pyruvate and lactate can be detected with high SNR for molecular imaging, including in a permanent stroke model13. Additionally, [1-13C]lactate can be readily hyperpolarized to study in-vivo metabolism14–16.
The present study relates to the potential of HP lactate and pyruvate as theranostic agents for transient ischemic stroke. To evaluate their potential at highlighting metabolic alterations following stroke, we measured the cerebral metabolism of a bolus of these HP compounds at their therapeutic dose in a transient stroke mouse model.
Methods
HyperpolarizationSodium L-[1-13C]lactate or [1-13C]pyruvic acid were hyperpolarized in a 7T/1K DNP polarizer17 with OX63 radical, yielding (35.7±11.5)% and ≈60%18 liquid-state polarization respectively.
Mouse middle cerebral artery occlusion (MCAO) stroke model
An ischemic lesion in the left striatum was induced by transient 30min MCAO in C57BL6/J male mice (n=17, 6-10 weeks) as previously described7,19. Stroke was considered successful if the regional cerebral blood flow was <20% during occlusion and >50% within 10min after reperfusion compared to baseline.
The left femoral vein was cannulated during occlusion for the HP solution injection. The control group was sham operated mice i.e. without suture insertion or artery ligation (n=9).
Acquisition
Upon reperfusion, mice were transferred into a 9.4T/31cm horizontal bore MRI scanner (Varian/Magnex) with a home-built 1H quadrature/13C single loop coil above the head. Each mouse was scanned only once, either 1h or 2h post-reperfusion in MCAO and 1h post-surgery in sham. A therapeutic dose of HP [1-13C]lactate (1.01±0.17umol/g) or [1-13C]pyruvate (1.15±0.12umol/g) was injected and 13C MR spectra were acquired every 3s with 30° BIR-4 adiabatic pulses.
Metabolite ratios
Metabolite peaks from the first 120s post-injection were fitted using the Bayesian Data-Analysis Software Package (Washington University in St. Louis).
Kinetic Modelling
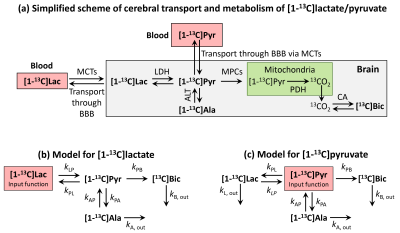
Metabolites time-courses were obtained from fitting each spectrum individually. Kinetic models were derived for [1-13C]lactate and [1-13C]pyruvate (Fig.1). Rate constants were determined by fitting each time course to the model using a trust-region least squares algorithm.
Statistical analysis
Variables are indicated as mean ± standard deviation. One-way ANOVA was used followed by Tukey-Kramer test. Significance level set to 0.05. Ratios and rates were normalized with the HP infusate dose for minimizing variation between individuals.
Results
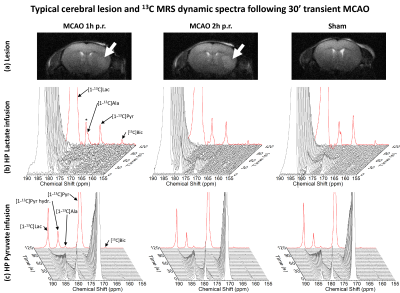
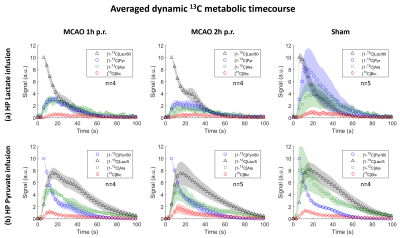
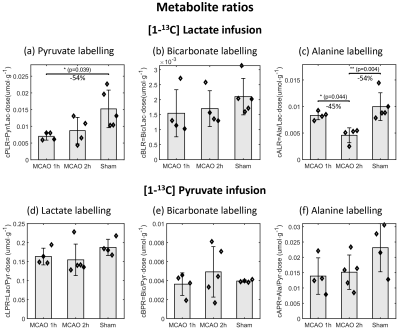
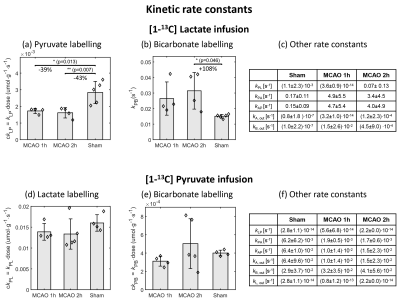
The striatal lesion evolves and becomes more contrasted on anatomical images at 2h compared to 1h post-reperfusion (Fig.2a). Metabolism of HP [1-13C]lactate or [1-13C]pyruvate was observed in healthy and stroke animals (Fig.2b-c, Fig.3).Following lactate infusion, the pyruvate-to-lactate ratio (cPLR) and the rate of lactate-to-pyruvate turnover (ckLP) were lower in MCAO compared to sham (Figs.4a-5a). After pyruvate injection, trends of lower lactate-to-pyruvate ratio (cLPR) and slower pyruvate-to-lactate rate constant (ckPL) are observed after stroke (Figs.4d-5d). Both substrates indicate lower interlabelling between lactate and pyruvate after stroke.
Significantly faster pyruvate-to-bicarbonate conversion after lactate injection is observed in MCAO 2h compared to sham (kPB, Fig.5b). This trend is also observed after pyruvate injection (ckPB, Fig.5e), and indicates higher mitochondrial activity.
Both substrates depict a trend towards lower labelling of alanine (Fig.4c and f). However, differences were only significant with HP lactate: the alanine-to-lactate ratio (cALR) is lower in MCAO 2h compared to MCAO 1h and sham, enabling distinguishing between both timepoints post-reperfusion.
Discussion
This study reports about real-time in-vivo measurements of global cerebral metabolism of the hyperpolarized neuroprotective agents [1-13C]lactate and [1-13C]pyruvate at their therapeutic dose after transient cerebral ischemia. While both substrates report on the same metabolic pathways and depicted similar trends of stroke-induced metabolic alterations, only with HP lactate those changes were significant.In both cases, the HP infusate signal prominently originates from the blood16,20. In HP lactate experiments, the labelling of pyruvate is directly related to the lactate transport across the BBB16, which is further metabolized into alanine and bicarbonate, enabling distinguishing between transport and metabolism. However, this is lacking in HP pyruvate experiments as blood and brain pyruvate cannot be distinguished.
Another advantage of using lactate is related to its higher endogenous concentration (≈20 times higher than pyruvate21). Therefore, lactate therapy stays closer to physiological conditions. Moreover, the flux of lactate across the BBB is higher than the one of pyruvate22.
The ability to probe several subsequent metabolic steps, higher flux through the MCTs and near physiological conditions contribute to the higher distinction observed between stroke and healthy animals while injecting HP lactate compared to pyruvate. This implies that HP lactate would potentially be a better theranostic biomarker for stroke. Our next step will be to differentiate between damaged and healthy tissues metabolism to further characterize the properties of those HP theranostic probes.
Acknowledgements
The authors gratefully thank Prof. Rolf Gruetter for supporting this collaboration, Drs. Analina Da Silva and Stefan Mitrea for their assistance in the animal preparation, as well as Drs. Hongxia Lei and Bernard Lanz for fruitful discussions. This study is supported by the Swiss National Science Foundation (310030_170155), the Centre d’Imagerie Biomédicale of the University of Lausanne, École polytechnique fédérale de Lausanne, University of Geneva, Geneva University Hospitals, Lausanne University Hospital, and the Leenaards and Louis-Jeantet Foundations.References
1. Johnson, W., Onuma, O., Owolabi, M. & Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 94, 634A-635A (2016).
2. Campbell, B. C. V. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 372, 1009–1018 (2015).
3. Saver, J. L. et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: Ameta-analysis. JAMA - J. Am. Med. Assoc. 316, 1279–1288 (2016).
4. Sandercock, P. et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 379, 2352–2363 (2012).
5. Bang, O. Y. Neuroprotective strategies for acute ischemic stroke: recent progress and future perspectives. Precis. Futur. Med. 1, 115–121 (2017).
6. Lei, H., Berthet, C., Hirt, L. & Gruetter, R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J. Cereb. Blood Flow Metab. 29, 811–819 (2009).
7. Castillo, X. et al. A probable dual mode of action for both L-and D-lactate neuroprotection in cerebral ischemia. J. Cereb. Blood Flow Metab. (2015). doi:10.1038/jcbfm.2015.115
8. Berthet, C., Castillo, X., Magistretti, P. J. & Hirt, L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: Extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc. Dis. (2012). doi:10.1159/000343657
9. Berthet, C. et al. Neuroprotective role of lactate after cerebral ischemia. J. Cereb. Blood Flow Metab. 29, 1780–1789 (2009).
10. Yi, J. S., Kim, T. Y., Kyu Kim, D. & Koh, J. Y. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol. Dis. 26, 94–104 (2007).
11. Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100, 10158–63 (2003).
12. Kurhanewicz, J. et al. Hyperpolarized 13 C MRI: Path to Clinical Translation in Oncology. Neoplasia 21, 1–16 (2019).
13. Xu, Y. et al. Hyperpolarized 13 C Magnetic Resonance Imaging Can Detect Metabolic Changes Characteristic of Penumbra in Ischemic Stroke . Tomography 3, 67–73 (2017).
14. Chen, A. P. et al. Feasibility of using hyperpolarized [1-13C]lactate as a substrate for in vivo metabolic13C MRSI studies. Magn. Reson. Imaging (2008). doi:10.1016/j.mri.2008.01.002
15. Bastiaansen, J. A. M., Yoshihara, H. A. I., Takado, Y., Gruetter, R. & Comment, A. Hyperpolarized 13C lactate as a substrate for in vivo metabolic studies in skeletal muscle. Metabolomics 10, 986–994 (2014).
16. Takado, Y. et al. Hyperpolarized 13 C Magnetic Resonance Spectroscopy Reveals the Rate-Limiting Role of the Blood-Brain Barrier in the Cerebral Uptake and Metabolism of l -Lactate in Vivo. ACS Chem. Neurosci. 9, 2554–2562 (2018).
17. Cheng, T. et al. Automated transfer and injection of hyperpolarized molecules with polarization measurement prior to in vivo NMR. NMR Biomed. 26, 1582–1588 (2013).
18. Yoshihara, H. A. I. et al. High-field dissolution dynamic nuclear polarization of [1-13C]pyruvic acid. Phys. Chem. Chem. Phys. 18, 12409–12413 (2016).
19. Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
20. Miller, J. J. et al. 13C Pyruvate Transport Across the Blood-Brain Barrier in Preclinical Hyperpolarised MRI. Sci. Rep. 8, 1–15 (2018).
21. Soto, M. et al. Pyruvate induces torpor in obese mice. Proc. Natl. Acad. Sci. U. S. A. 115, 810–815 (2018).
22. Rodrigues, T. B., Sierra, A., Ballesteros, P. & Cerdán, S. Pyruvate Transport and Metabolism in the Central Nervous System. in Neural Metabolism In Vivo (eds. Choi, I.-Y. & Gruetter, R.) 715–753 (Springer US, 2012). doi:10.1007/978-1-4614-1788-0_24
Figures