0688
Hyperpolarized [1-13C] dehydroascorbic acid imaging of ascorbate-mediated oxidative stress in pancreatic cancer1Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Antitumor Assessment Core Facility, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Weill Cornell Medical College, New York, NY, United States, 4Meyer Cancer Center, Weill Cornell Medical College, New York, NY, United States
Synopsis
We investigated hyperpolarized [1-13C] dehydroascorbic acid (HP DHA) as an imaging agent for probing oxidative stress in patient derived xenograft models (PDXs) of pancreatic cancer. By increasing the T1 via D2O solvation and increasing the dose administered via awake mouse injection, conversion of DHA to ascorbate was readily observed in BRAF and KRAS mutant cancers. HP DHA was then used to characterize oxidative stress in these PDX models and their biochemical mechanism of response to ascorbate therapy. Changes in DHA/ascorbate metabolism were measured in these tumor models, demonstrating a proof of concept method for assessing ascorbate therapy in pancreatic cancer.
Introduction
Tumor progression and survival is driven by genetic mutations and environmental conditions. Recent work has revealed that redox metabolism is deregulated in pancreatic cancer and may provide new opportunities for diagnosis and targeted treatment. Specifically, a therapeutic strategy for targeting KRAS mutant cancers using high dose ascorbate was introduced to disrupt redox homeostasis.1 Ascorbate can be oxidized in the presence of Reactive Oxygen Species (ROS) to form dehydroascorbate (DHA), which is similar in molecular structure to glucose and can therefore be efficiently taken up by glucose transporter (GLUT1). GLUT1 is overexpressed in KRAS mutant cancer cells and other oncogene-driven tumor models. After entering the cell, DHA is reduced back to ascorbate in a process that consumes the antioxidant glutathione, which causes a depletion of the intracellular redox machinery and in turn sensitizes cells to ROS. Each successive turn of this process results in a cyclical increase in oxidative stress in the tumor microenvironment. While targeted therapeutic strategies have been introduced, methods to monitor the metabolic reprogramming of redox homeostasis are critically needed. We aim to identify hyperpolarized DHA (HP DHA) as an imaging agent for quantifying oxidative stress in pancreatic cancer. While HP DHA has been developed to interrogate redox capacity in tumors,2 its clinical and preclinical use has been hampered due to its short T13 and possible toxicity at high doses. We sought to address both these limitations to demonstrate its use in monitoring oxidative stress in patient derived xenograft models (PDXs) of pancreatic cancer.Methods
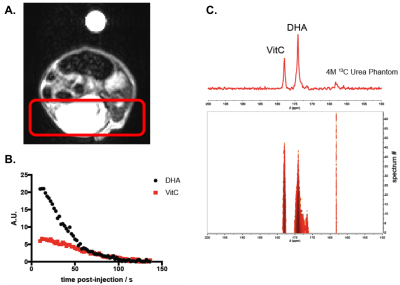
Hyperpolarized probe and in vivo optimization – 30 μL of 2.5 M [1-13C] DHA in dimethylacetamide were dissolved in 10 mL of 0.3 mM EDTA in D2O. The solution of HP DHA was subjected to a 1T magnet to measure the effect of D2O solvation on the T1 of HP DHA.4 All animal studies were conducted under an IACUC approved animal protocol. A panel of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were injected intravenously with increasing doses of DHA to determine the feasible limit of toxicity for hyperpolarized MRS studies. NSG mice were found to tolerate 50 mM DHA when injected awake, a dose much greater than the 7 mM DHA tolerated when anesthetized under isoflurane.Increased lifetime of HP DHA in vivo – HP DHA was injected intravenously into a subcutaneous xenograft of an HCT116 tumor model in NSG mice. MRS of HP DHA using a slab dynamic acquisition revealed the lifetime of the hyperpolarized signal observed of DHA and ascorbate in the tumor as well as the relevant NMR parameters (flip angle and repetition time for exciting the products and temporal resolution).
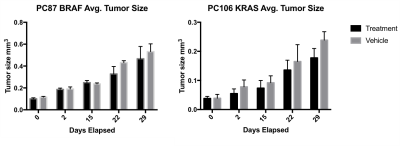
Ascorbate therapy of pancreatic cancer PDXs – BRAF-driven and KRAS-driven PDX models, created from patient samples and verified using targeted IMPACT sequencing,5,6 were subcutaneously xenografted in the right flank of NSG mice. 2 weeks after implantation, mice were randomly divided into two groups. One group was treated with freshly prepared vitamin C in 400 μL of PBS (4 g/kg) twice a day via IP injection (PC87, n= 4; PC106 n = 5). Control group mice were treated with PBS using the same twice a day dose (PC87, n = 5; PC106 n = 5). T2 images of these mice were acquired weekly to quantify tumor size and evaluate changes in tumor growth over time.
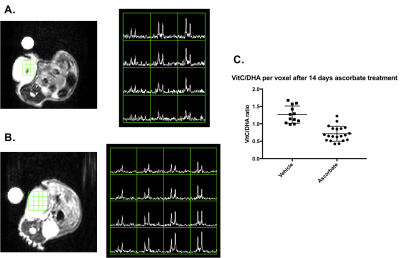
HP DHA imaging of pancreatic cancer PDXs – Ascorbate and vehicle-treated mice of both tumor models were injected with 250 μL of 20 mM HP DHA in 0.3 mM EDTA in D2O to investigate the redox status of the implanted tumor over time. 13C HP MRS was performed using an axial 2D CSI acquisition on a 5T small animal MRI spectrometer to measure DHA/ascorbate metabolism. DHA/ascorbate ratios were quantified by taking the area under the curve and compared between ascorbate-treated and vehicle mice to determine changes in tumor redox upon ascorbate therapy.
Results and Discussion
As anticipated, increasing the T1 of HP DHA via D2O solvation (67.5 ± 4.5 s to 83.4 ± 5.7 s) and the concentration administered via awake mouse injection (7 mM to 20 mM) allowed for the prolonged hyperpolarized signal of DHA and its metabolic product, ascorbate, in tumors (Figure 1). Ascorbate treatment was shown to significantly slow tumor growth in a KRAS-driven PDX model after 4 weeks while the effect on tumor growth was not significant in a BRAF-driven PDX model (Figure 2). Interestingly, while there was no change observed in the HP DHA/ascorbate ratio in vivo after 1 day of treatment in the BRAF PDX (data not shown), the HP DHA/ascorbate ratio was found to have changed after 2 weeks of ascorbate treatment (Figure 3a and 3b). This change in redox could be measured either by taking the total DHA and total ascorbate signal for the whole tumor or comparing the quantified signal per voxel in the ascorbate and vehicle-treated mice (Figure 3c).Conclusion
We demonstrated HP DHA as a biomarker for measuring changes in redox metabolism in PDX models of pancreatic cancer. Changes in HP DHA/ascorbate signals were observed and quantified after prolonged therapy, which may be indicative of oxidative stress in the tumor upon ascorbate treatment.Acknowledgements
The authors acknowledge the Stand Up to Cancer Foundation, Starr Cancer Consortium, Thompson Family Foundation and NIH/NCI Cancer Center Support Grant P30 CA008748 for funding and support.References
1. Yun, J., Mullarky, E., Lu, C., Bosch, K. N., Kavalier, A., Rivera, K., Roper, J., Chio, I. I. C., Giannopoulou, E. G., Rago, C., Muley, A., Asara, J. M., Paik, J., Elemento, O., Chen, Z., Pappin, D. J., Dow, L. E., Papadopoulos, N., Gross, S. S., & Cantley, L. C. (2015). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350(6266), 1391-1396. doi:10.1126/science.aaa5004.
2. Keshari, K. R., Sai, V., Wang, Z. J., Vanbrocklin, H. F., Kurhanewicz, J., & Wilson, D. M. (2013). Hyperpolarized [1-13C]dehydroascorbate MR spectroscopy in a murine model of prostate cancer: comparison with 18F-FDG PET. Journal of Nuclear Medicine, 54(6), 922–928. doi:10.2967/jnumed.112.115402.
3. Keshari, K. R., Kurhanewicz, J., Bok, R., Larson, P. E., Vigneron, D. B., & Wilson, D. M. (2011). Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America, 108(46), 18606–18611. doi:10.1073/pnas.11069201084.
4. Cho, A., Eskandari, R., Miloushev, V. Z., & Keshari, K. R. (2018). A non-synthetic approach to extending the lifetime of hyperpolarized molecules using D2O solvation. Journal of Magnetic Resonance, 295, 57–62. doi:10.1016/j.jmr.2018.08.0015.
5. Cheng, D. T., Mitchell, T. N., Zehir, A., Shah, R. H., Benayed, R., Syed, A., … Berger, M. F. (2015). Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of Molecular Diagnostics, 17(3), 251–264. doi:10.1016/j.jmoldx.2014.12.0066.
6. Mattar, M., McCarthy, C. R., Kulick, A. R., Qeriqi, B., Guzman, S., & de Stanchina, E. (2018). Establishing and Maintaining an Extensive Library of Patient-Derived Xenograft Models. Frontiers in Oncology, 8, 19. doi:10.3389/fonc.2018.00019.
Figures