0668
An improved spiral technique for imaging gamma knife subject with metal frame1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States, 2St Joseph's Hospital and Medical Center, Phoenix, AZ, United States
Synopsis
SSFP is widely used in gamma knife treatment planning in patients with trigeminal neuralgia. A metal frame worn by the patient often causes image artifacts. A spiral FLAIR technique is proposed with an improved approach for concomitant field induced phase error compensation, which is insensitive to the presence of the metal frame. Volunteer results demonstrate good delineation of the trigeminal nerve root entry zone, good adjacent CSF suppression, and good brain stem tissue contrast, therefore providing a potential alternative to SSFP for gamma knife treatment planning.
Introduction
Trigeminal neuralgia is a chronic disease due to the malfunction of the trigeminal nerve (TN), typically caused by compression at the TN root entry zone. Surgical treatment options primarily include microvascular decompression (MVD) and stereotactic radio surgery (SRS) with a gamma knife. In SRS, T1 and SSFP (FIESTA-C, CISS, 1 balanced-FFE) MRI are most commonly used and generally sufficient for targeting the TN. 2,3 However, in some patients the TN is not clearly delineated due to artifacts, especially in SSFP, with the presence of a metal frame worn by patients for treatment planning (illustrated with a skull phantom in Fig. 1). In addition, tissue contrast with SSFP is poor. Alternatives, such as traditional Cartesian T2 and FLAIR imaging, may not be robust due to the metal frame. In this work, we improve a spiral FLAIR technique 4 with a concomitant phase compensation (CPC) approach that is insensitive to field perturbation caused by the metal frame, to provide better visualization of the TN with good CSF suppression, and good contrast in the brain stem.Methods
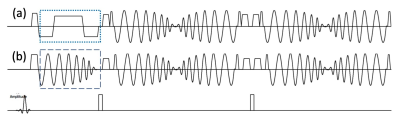
A 3D spiral FLAIR technique has been developed to provide high SNR, good CSF suppression, and minimal flow artifacts, compared to 3D Cartesian FLAIR. 4 The 3D spiral FLAIR sequence uses a group of flow compensated gradients to compensate for the concomitant field induced phase errors (dotted box in Fig. 2a). However, these trapezoidal gradients resemble EPI readout gradients and are therefore sensitive to field disturbance due to the presence of the metal frame and other sources of field variations. To mitigate this issue, we propose to use spiral gradients (dashed box in Fig. 2b) with smooth transitions and hence less sensitive to field change as an alternative for CPC.The spiral FLAIR sequence was implemented on a Philips 3T Ingenia scanner (Philips Healthcare, Best, the Netherlands) with a T/R head coil. Volunteers wearing the metal frame were scanned using spiral FLAIR with the proposed CPC method to demonstrate its feasibility. Imaging parameters include: FOV = 230x230x92 mm3, acquisition resolution = 1.2x1.2x2 mm3, ETL = 50, TR = 4800 ms, TI = 1500 ms, scan time = 6:29. For comparison, data were also acquired using spiral FLAIR with original CPC approach, Cartesian T2 and FLAIR (Brainview T2 and FLAIR), and SSFP (b-FFE).
Results and Discussions
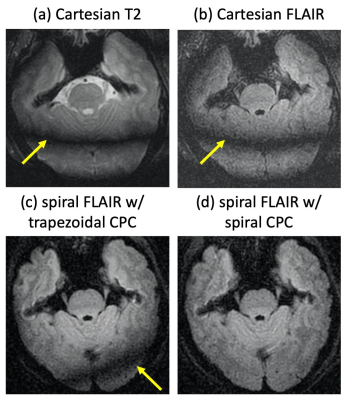
Fig. 3 shows 3D T2 and FLAIR images as well as the spiral FLAIR data with original trapezoidal gradients and the proposed spiral gradients for CPC. Strong banding/shading artifacts are observed in Cartesian T2/FLAIR and spiral FLAIR with trapezoidal CPC gradients. In spiral FLAIR with the proposed spiral gradients for CPC, these artifacts are significantly reduced, demonstrating the effectiveness of the spiral CPC gradients.With the spiral gradients for CPC, the echo spacing and therefore the echo train duration does increase, which slightly decreases the SNR (Figs. 3c vs 3d). However, this impact is insignificant compared to the reduction of banding artifacts with the original approach.
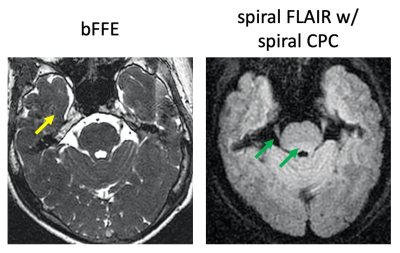
Fig. 4 compares an SSFP image with the spiral FLAIR result. SSFP relies on the high contrast between bright CSF and brain tissues to identify the TN, but typically has low contrast between brain tissues, and is often degraded by flow artifacts. The TN is better visualized in spiral FLAIR due to excellent suppression of the adjacent CSF. In spiral Flair, the brain stem tissue contrast is also improved, and flow artifacts are significantly minimized. The artifacts due to field disturbance sometimes seen in SSFP data are not present in the volunteer images but further study on a large cohort of volunteers/patients is required. Future study also includes investigation of the geometric accuracy of spiral FLAIR and protocol optimization.
Conclusion
In summary, the proposed spiral FLAIR technique with a spiral gradient for concomitant phase compensation demonstrates insensitivity to field disturbance due to the metal frame used in gamma knife treatment planning, good delineation of the TN, and improved brain stem tissue contrast, providing a potential alternative to SSFP for gamma knife treatment planning in patients with trigeminal neuralgia.Acknowledgements
This work is partially supported by Philips Healthcare.References
1. Deimling M, et al. Constructive interference in steady state (CISS) for motion sensitivity reduction. In the proceeding of SMRM 1989:842.
2. Yoshino N, et al. Trigeminal neuralgia: Evaluation of neuralgic manifestation and site of neurovascular compression with 3D CISS MRI imaging and MR angiography. Radiology 2003;228:539-545.
3. Haller S, et al. Imaging of neurovascular compression syndromes: Trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. AJNR Am J Neuroradiol 2016;37:1384-1392.
4. Li Z, et al. Improving the image quality of 3D FLAIR with a spiral MRI technique. Magn Reson Med 2019;83:170-177.
Figures