0664
Edge-preserving B0 inhomogeneity distortion correction for high-resolution multi-echo ex vivo MRI at 7T
Divya Varadarajan1,2, Robert Frost1,2, Andre van der Kouwe1,2, Leah Morgan1, Bram Diamond1, Emma Boyd1, Morgan Fogarty1, Allison Stevens1, Bruce Fischl1,2, and Jonathan R Polimeni1,2,3
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Harvard-MIT HST, Cambridge, MA, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Harvard-MIT HST, Cambridge, MA, United States
Synopsis
High-resolution multi-echo MRI at ultra-high field for ex vivo imaging is time consuming, SNR starved and suffers from B0 inhomogeneity induced geometric distortions due to low-bandwidth in the readout direction. Fieldmap-based correction cannot correct singularities in regions of severe distortion, and reversed gradient (RG) approaches double the scan time. We propose to combine an alternating-polarity acquisition scheme for multi-echo MRI with a low-resolution fieldmap based novel distortion correction algorithm that can correct singularities in half the scan time of RG and enhance SNR while preserving edges. We show several ex vivo corrected results and demonstrate generalizability to in vivo MRI.
Introduction
B0 nonuniformity due to susceptibility inhomogeneity causes geometric distortions in MRI whenever B0 offsets are large relative to the encoding bandwidth (BW). This is well studied for EPI acquisitions1-4. However, here we focus on a lesser-known case of high-resolution (100–150 μm), ultra-high field (7T), multi-echo ex vivo MRI, where air-tissue interfaces (e.g. air bubbles) induce strong B0 offsets, and the low-BW required to achieve high-resolution makes this data vulnerable to distortions spanning 100–1000 μm along the readout axis5.Ex vivo MRI is used to study micro-architectonics, where even distortions at 100 μm are problematic5. Conventional fieldmap1,2 methods cannot resolve singularities in the image such as pile-up. Reversed-gradient3-4,6 (RG) methods can resolve singularities but require double the scan time because two images with matching contrast and opposing distortions must be acquired. Long scan times of ex vivo imaging make both acquiring a high-resolution fieldmap or an additional RG scan impractical, rendering existing methods unusable. Timing constraints often prevent the acquisition of multiple averages, resulting in SNR-starved data.
In order to overcome these challenges, we propose a novel acquisition and optimization framework that encodes opposing distortions in a single multi-echo scan, incorporates a low-resolution fieldmap, and jointly corrects all echoes using a shared edge constraint that enhances SNR and corrects distortion singularities without additional high-resolution scans. We show several ex vivo results and demonstrate generalizability to in vivo MRI.
Methods
Our acquisition consists of a multi-echo gradient-echo pulse sequence where we encode alternating echoes with opposite readout directions, positive and negative; opposite readouts cause the distortions to be in opposing directions in consecutive echoes to improve SNR efficiency7. Using a forward signal model7 we synthesize the opposite polarity for every echo. That is, in a four-echo acquisition in which TE1 is acquired with RO+, TE2 with RO−, TE3 with RO+, and TE4 with RO−, we use echoes at TE2 and TE4 to synthesize an image at TE3 with RO−. This ensures we have both readout polarities for all contrasts and allows us to correct for singularities using a single multi-echo acquisition.Our method then jointly corrects distortion in all echoes by solving a cost function given by, $$\arg\min_{\rho_0 \cdots \rho_N} \sum_{i=1}^N{\|K_i\rho_i-\tilde{y}\|^2+\lambda\|diag(\sqrt{\ell_i})D\rho_i\|^2},$$ where $$$N$$$is the number of echoes, $$$i$$$ is an echo index, $$$\rho_i$$$ is the undistorted echo, $$$y_i$$$ is the acquired (distorted) echo and $$$\tilde{y_i}$$$ concatenates the acquired and the synthesized opposite polarity echo. The first term is a least-squares data fidelity where $$$K_i$$$ contains the linear shift due to B0 inhomogeneity. We use a low-resolution fieldmap to calculate $$$K_i$$$. The regularization term assumes that echoes share the same edge locations corresponding to anatomy8. Here, $$$\ell_i$$$ is the line process prior9 calculated using the $$$L_{2,1}$$$ norm indicating group sparsity i.e. common edges across echoes and $$$D$$$ is the finite difference operator. The line process will be close to one in smooth regions of the image, reducing the regularizer to a quadratic smoothness term that enhances SNR. The regularization vanishes at common edges due to low values of $$$\ell_i$$$ thereby preserving them. The optimization was solved using conjugate gradient.
We tested the proposed method by acquiring three ex vivo human brain hemisphere datasets with a 4-echo protocol: two at 150 μm (TE=[5.65,11.95,18.25,24.55] ms, TR =34 ms, FA=20°) and one at 100 μm (TE=[7.5,15.5,23.5,31.5] ms, TR=45 ms, FA=10°). A 2-mm resolution fieldmap was additionally acquired in all sessions. We tested generalizability of the proposed method to in vivo multiecho MPRAGE (TE=[1.61,3.47,5.33,7.19] ms, TR=2.53 s, FA=7°) data at 1 mm resolution.
Results
Figures 1 and 2 show results of applying the proposed method to the 150 μm ex vivo dataset. Figure 1 zooms into the cerebellum (top row) and frontal lobe (bottom row) with inhomogeneity of 100–650 μm. Figures 1c–f show the root-mean-square (RMS) combination across all echoes and edge maps, with red and green edges corresponding to echoes 0 and 1 respectively and yellow edges are regions of perfect alignment between the echoes. RMS images of uncorrected data in Figs. 1c and e are blurry or have unaligned edges (see arrows), while RMS images of the corrected data in 1d. and 1f. are sharper with well-aligned edges. Figure 2 compares the proposed framework with FSL’s topup4 applied to conventional RG data that require twice the scan time to acquire. Both methods correct distortion well (blue arrow), but the proposed method exhibits improved edge preservation (purple arrow) and requires half the acquisition time. Figure 3 shows a 100 μm sagittal slice through visual cortex of the third echo, and edge maps of the third and fourth echoes (in red and green) in a region of strong field inhomogeneity. The edges align well after correction, and SNR is considerably improved without blurring the anatomy. Figure 4 shows an in vivo MEMPRAGE coronal slice with distorted temporal lobe. Distortion is visible in 4b edge map where the consecutive echoes do not align. The proposed method corrects the distortion well and accurately aligns the echoes.Conclusion
We presented a novel distortion correction framework for high-resolution, 7T multi-echo ex vivo data that enhances SNR and optimizes acquisition time. We also demonstrated that it extends to routine in vivo MRI.Acknowledgements
This work was supported in part by the NIH NIBIB (grants P41-EB015896), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023043 and S10-RR019371.References
- Jezzard, P. & Balaban, R. S. Correction for geometric distortion in echo planar images from Bo field variations. Magn. Reson. Med. 34, 65-73 (1995).
- Bhushan, C., Joshi, A. A., Leahy, R. M. & Haldar, J. P. Improved B0 -distortion correction in diffusion MRI using interlaced q-space sampling and constrained reconstruction. Magn Reson Med 72, (2014).
- Chang H, Fitzpatrick JM. A technique for accurate magnetic resonance imaging in the presence of field inhomogeneities. IEEE Trans Med Imaging, 11: 319–329 (1992).
- Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870-888, (2003).
- Polimeni J.R., Renvall V., Zaretskaya N., Fischl B., Analysis strategies for high-resolution UHF-fMRI data, Neuroimage, 168, 296-320,(2018)
- Robert Frost, Divya Varadarajan, Hui Wang, Jesper LR Andersson, Emma Boyd, Lee Tirrell, Bram Diamond, Leah Morgan, Allison Stevens, Jonathan R Polimeni, Bruce Fischl, André JW van der Kouwe. In Proceedings of the 27th Annual Meeting of OHBM, Rome, Italy,(2019)
- Fischl, B., Salat, D., van der Kouwe, A., Makris, N., Ségonne, F., Quinn, B. & AM., D. Sequence-Independent Segmentation of Magnetic Resonance Images. Neuroimage 23, S69–S84, (2004).
- Haldar J. P. , Wedeen V. J., Nezamzadeh M., Dai G., Weiner M. W., Schuff N., Liang Z.-P.. “Improved Diffusion Imaging through SNR-Enhancing Joint Reconstruction.” Magnetic Resonance in Medicine 69:277–289, (2013).
- Geman S. and Geman D., “Stochastic relaxation, Gibbs distribution, and the Bayesian restoration of images,” IEEE Trans. Patt. Anal. Mach. Int., vol. 6, pp. 721–741, (1984).
Figures
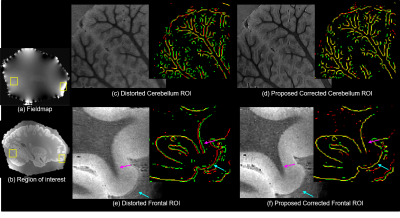
Figure1. B0
Distortion correction at 150 micron: Images show the RMS across echoes of distorted
data and result of proposed method in the cerebellum and frontal cortex. Edge
maps (red and green - individual echo edges, yellow - perfect overlap) further
demonstrate improved alignment between echoes post correction.
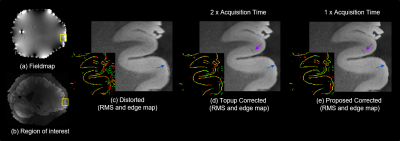
Figure 2. Optimizing
acquisition time: Images show RMS across echoes of distorted data and results
of correcting with FSL’s topup applied to reversed gradient data, and the proposed
method applied to half the data as topup. Proposed method matches topup in
performance (blue arrow), enhancing edges marginally using half the acquisition
time. Edge maps (red and green - individual echo edges, yellow - perfect overlap) show good alignment in both topup and proposed approach.
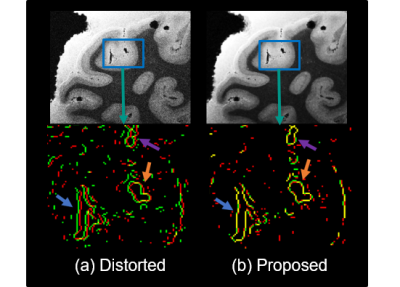
Figure 3. SNR enhancement at 100 micron: Images show
the SNR improvement after correction in the visual cortex with the proposed
method at 100 micron resolution. Edge maps (red and green - individual echo edges, yellow - perfect
overlap) further demonstrate improved alignment between echoes post
correction.
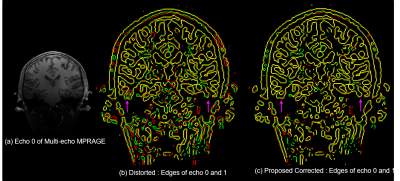
Figure 4. In-vivo
multi-echo MPRAGE B0 Distortion correction:
Results show edges of first two echoes of a MEMPRAGE scan in red and
green. Temporal lobe is the most affected by distortion (see arrows), which the
proposed method is able to resolve successfully seen in the edge maps as improved
alignment.