0663
Multiple phase unwrapping of 4D-flow MRI in cardiovascular valves and vessels1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 3Department of Surgery and Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Synopsis
Accurate quantification of both high and low blood velocities is important for clinical decision-making in cardiovascular conditions like aortic valve stenosis. Multi-VENC acquisition is a potential solution. An alternative, without increasing acquisition time, is enabled here by correcting 4D-Flow MRI multi-aliasing along tubular structures. Our solution is based on continuity principles in successive cross-section planes, outperforming the state-of-the-art Laplacian-based solution which only performs single-wrap corrections. The accuracy of proposed method is verified in 4D-Flow MRI of 25 aortic stenotis patients with VENC of 1m/s, where double and triple unwrapping were needed to match velocity values measured by Doppler echocardiography.
Introduction
Aortic valve stenosis (AVS) is the most common valvular heart disease, affecting 3% of the world population and increasing with population ageing.1 AVS consists of a diminished open valve orifice area resulting in an increased trans-valvular velocity during ejection and thus in an increased pressure drop (DP). Intra-ventricular heamodynamics are also altered in AVS and are reported to provide complementary information for clinical decision making.2,3 There is thus a need to acquire simultaneously and with high accuracy very high trans-valvular velocities (>3m/s) and relatively low intraventricular velocities (±0.5m/s).4D-flow MRI, with dual or multi- velocity encoding (VENC) is the current strategy to meet this need of wide velocity range. However, this causes an increase in the acquisition time (and thus cost), and this technique is still not widely available. The other would be a single VENC acquisition with an adequate phase unwrapping, but current solutions including the well-praised Laplacian‐based solution only allow for a single wrap correction.4 We thus hypothesised that it is possible to correct multi-aliasing (e.g. double or triple) from a low VENC 4D-flow acquisition in a tubular cardiovascular structure following the principle of continuity and mass conservation.
Methods
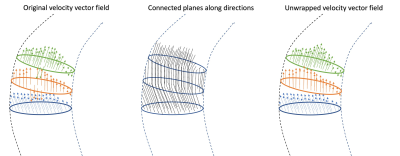
4D-flow MRI was acquired in 25 AVS patients with a VENC of 1m/s, 30 frames per heart-cycle, isotropic spatial resolution of 3mm, and field-of-view containing the left ventricle (LV) and the full thoracic aorta (ascending, aortic arch and thoracic descending). Semi-automatic segmentation of left ventricular outflow tract (LVOT) and aorta was performed based on the average systolic frames of 4D-flow MRI. The centreline along the segmented vessel was computed and sampled at points every 1 mm. For each centreline point, a geometric cross-section of the vessel was generated and velocity vectors of intersected voxels are assigned to each cross-plane the cross-plane.Multi-aliasing correction is then performed by comparing consecutive cross-sections and testing for the number of unwraps needed to best conserve momentum between them. Considering that the LVOT segmentation was conveniently started in the mid-LV, where the velocities are lower than 1m/s, it is verified that there is no aliasing in the first plane. Comparison is based on the calculation of a coefficient of likelihood for: no aliasing, single, double and triple aliasing per each of the 3 velocity components and per each voxel in-plane (see Figure 1). After the plane correction, the gaps between two consecutive planes are assessed and in case there were skipped voxels, they will be corrected based on the neighbour voxels in both planes.
Evaluation is performed by comparing physical magnitudes of flow and pressure drop before and after multi-unwrapping. Pressure drop is computed by the Simplified Advective Work-energy relative pressure formulation (SAW).5 Validation is performed by comparing the resultant peak and mean velocities with the same measurements obtained by Doppler echocardiography.
Results
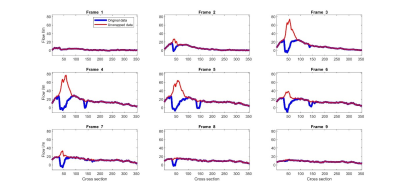
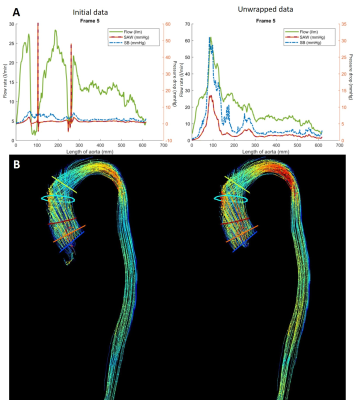
Corrected peak and mean velocities (2.95±0.45m/s) are almost equivalent to the peak velocities obtained by echocardiography (2.60±0.27 m/s) and show quasi-equivalence (y = 0.72x + 1.14 and mean difference between measurements of 0.35 m/s and confidence intervals of 0.7 m/s and -0.01 m/s). On the other side, original data with known aliasing (1.39±0.10 m/s) is significantly different than the echocardiography velocities (y = 0.17x + 1.11 and mean difference between measurements of 1.56 m/s and confidence intervals of 2.2 m/s and 0.8 m/s). Visually it can also be assessed the improved velocity vector field (Figure 2 and 4B). Finally, flow rate and SAW provide smoother profiles over the aorta length in comparison with multi-wrapped images (Figures 3 and 4A).Discussion
This study introduces for the first time a fully automated method to correct multi-aliasing in 4D-Flow MRI on cardiovascular vessels and valves.The state-of-the-art solution is a one-shot that corrects the full FOV based on the Laplacian of the phase, but in practice fails when trying to detect multiple wraps. The open-source code applied to the present datasets did not correct the data for more than one phase wrap (see Figure 5).4
There were small differences when comparing unwrapped MRI and Doppler-echocardiography peak velocities and they can be explained by echocardiography acquisition limitations: velocities are obtained in the single optimal direction found, and depend on the window of the patient’s chest and shadowing (presence of calcifications on the valve). It is, therefore, possible that the peak event can be missed.
Our solution is based on cross-section planes, and assumes the continuity of momentum between adjacent planes, and as such is not applicable to any velocity field. Further work is needed to refine and test the multi-unwrapping performance when dealing with heart cavities with big vortical flow structures and splitting flow such as jets hitting on the wall or vascular bifurcations. Nevertheless, in both these scenerios, there is less prospect of occurring double-aliasing.
Conclusion
The present study shows for the first time a methodology for correction of 4D-Flow MRI multi-aliasing in cardiovascular vessels. This is relevant to decrease the acquisition time (factor of at least 2) in 4D-Flow MRI when a wide range of velocity values are desired.Acknowledgements
JFF: PIC project, European Union’s Horizon 2020 Marie Skłodowska-Curie ITN Project under grant agreement No 764738. PL holds a Wellcome Trust Senior Research Fellowship (g.a. 209450/Z/17/Z).References
1. Thaden JJ, Nkomo VT, Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis 2014;56:565-71.
2. Dyverfeldt P, Kvitting JPE, Sigfridsson A et al. Assessment of fluctuating velocities in disturbed cardiovascular blood flow: in vivo feasibility of generalized phase‐contrast MRI. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine 2008;28:655-663.
3. von Knobelsdorff-Brenkenhoff F, Karunaharamoorthy A, Trauzeddel RF et al. Evaluation of aortic blood flow and wall shear stress in aortic stenosis and its association with left ventricular remodeling. Circ Cardiovasc Imaging 2016;9:e004038.
4. Loecher M, Schrauben E, Johnson KM, Wieben O. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016;43:833-42.
5. Donati F, Myerson S, Bissell MM et al. Beyond Bernoulli: Improving the Accuracy and Precision of Noninvasive Estimation of Peak Pressure Drops. Circ Cardiovasc Imaging 2017;10.
Figures