0661
PhysiCal: A rapid calibration scan for B0, B1+, coil sensitivity and Eddy current mapping.1Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3MR Collaborations, Siemens Healthcare Ltd, Shanghai, China
Synopsis
Calibration scans that acquire coil sensitivity, B0 and B1+ inhomogeneities information play an important role in enabling modern acquisition and reconstruction techniques. This work proposes a unified, rapid calibration sequence termed Physics Calibration (PhysiCal) to obtain accurate B0, Eddy, B1+ and coil sensitivity maps. PhysiCal utilizes a carefully designed mix of full and variable density sampling acquisitions across echoes with synergistic constrained and eigenvalue reconstruction for robust and accurate recovery of whole-brain B0, B1+, Eddy and 32-channel coil sensitivity maps in just 11 seconds at 1 mm x 2 mm x 2mm resolution at 3T.
Introduction.
Calibration scans for coil sensitivity-maps (CSM),$$$\,B_0\,$$$and$$$\,B_1^+\,$$$inhomogeneities information play an important role in enabling modern acquisition and reconstruction techniques. Tremendous progress has been made in improving the accuracy and speed of these scans. For CSM, ESPIRiT1 and JSENSE2 have been successful in enabling wide-spread parallel imaging, while for accelerated-EPI, FLEET-ACS3 and Gradient Echo (GRE) with$$$\,B_0\,$$$field map4 have provided distortion-matched CSM for robust reconstruction. For$$$\,B_1^+\,$$$mapping, Bloch-Siegart5 (BS) methods have gained prominence due to its flexibility and robustness, where a recent improvement6 through k-space under-sampling and constrained-reconstruction has enabled rapid$$$\,B_1^+\,$$$mapping. Moreover, a robust multi-echo general linear modelling (GLM) framework for BS7 has also been developed that enables robust recovery$$$\,B_0$$$,Eddy and$$$\,B_1^+\,$$$maps.Nonetheless, the acquisition of multiple calibration scans for high-resolution coil sensitivity,$$$\,B_1^+\,$$$and$$$\,B_0\,$$$maps can be time consuming, taking$$$\,5-10\,$$$minutes for whole-brain coverage. This work proposes a unified, rapid calibration sequence termed Physics Calibration (PhysiCal) to obtain accurate$$$\,B_0$$$, Eddy,$$$\,B_1^+\,$$$and CSM. PhysiCal utilizes a modified BS multi-echo GRE acquisition, with a carefully designed mix of full and variable density sampling acquisitions across echoes to provide complementary information. This along with synergistic constrained and eigenvalue reconstructions enable$$$\,\sim50\times\,$$$speedup of this calibration scan. Retrospective under-sampling experiments demonstrate robust and accurate recovery of whole-brain$$$\,B_0$$$,$$$\,B_1^+$$$, Eddy and$$$\,32-$$$channel coil sensitivity maps in just 11 seconds at$$$\,1mm\times 2\,mm\times2\,mm\,$$$resolution at 3T. Furthermore, preliminary verification of PhysiCal is presented through using the rapidly generated$$$\,B_0\,$$$and$$$\,B_1^+\,$$$ maps to process high-resolution diffusion-imaging data acquired with accelerated gSlider-EPI8,9.
Acquisition and Reconstruction.
Figure 1 summarizes PhysiCal acquisition. A modified BS, bi-polar, multi-echo 3D-GRE acquisition is used with interleaved, opposite, off-resonant frequencies$$$\,(\pm4\,kHz)$$$10, and with multi-echo readouts prior and subsequent to the BS pulse6 to achieve estimation robustness. The k-space sampling of this acquisition is optimized with:- A small fully-sampled$$$\,(k_y, k_z)=(16,16)\,$$$auto-calibrated signal (ACS) in the first echo for ESPRIT-CSM.
- Independently drawn variable density Poisson-disc sampling across echoes$$$\,(\sim50\times)$$$.
Figure 2 depicts the reconstruction. ESPIRiT is used to calibrate CSM from ACS of the first echo. CSM is then used to perform an$$$\,\,l_1$$$-Wavelet regularized PI+CS reconstruction of highly under-sampled echo data$$$\,(\sim50\times)$$$. Subsequently, GLM robustly recovers artifact-free$$$\,B_0\,$$$and Eddy from reconstructed echo images (which contain temporally-incoherent residual artifacts). However, GLM cannot recover artifact free$$$\,B_1^+$$$. To overcome this, $$$B_1^+$$$ is refined using an eigenvalue reconstruction approach inspired by ESPIRiT. Phase of the reconstructed echoes after the negative-offset BS pulse is subtracted from the phase of the reconstructed echoes from the positive-offset BS echoes. The resulting time-series is reshaped into “virtual coils” and passed into ESPIRiT, which recovers the expected smooth underlying$$$\,B_1^+$$$.
Methods.
Data Acquisition: Fully-sampled$$$\,1\times2\times2\,mm^3\,$$$resolution PhysiCal data with$$$\,16\,$$$echoes (4 before BS) are acquired in 19 minutes and 41 seconds$$$\,(\text{TR}:\,36 ms,\,\text{echo-spacing:}\,1.25 ms)$$$. Gold standard$$$\,B_0,B_1^+\,$$$and Eddy maps are estimated using GLM and CSM is estimated from the first GRE echo using ESPIRiT.Acceleration Experiments: Acquired data are retrospectively under-sampled by varying the number of sampled$$$\,(k_y,k_z)\,$$$points per echo and the number of echoes. First echo contains$$$\,16\times16\,$$$ACS for ESPIRiT-CSM.$$$\,B_0, B_1^+$$$, Eddy and CSM are recovered through the procedure outlined above, and are then compared to the gold standard. BART11 was used for sampling-mask generation, ESPIRiT and PI+CS.
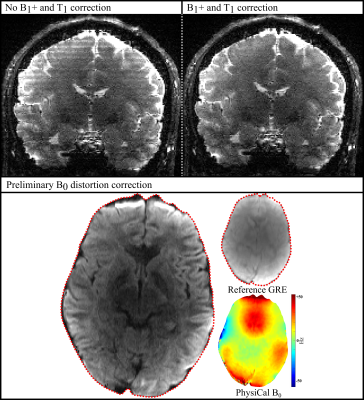
Preliminary Efficacy Verification: To demonstrate the applicability, accelerated parametric maps obtained from PhysiCal are used in EPI-gSlider. $$$B_1^+\,$$$inhomogeneity is used to mitigate striping artifacts in an EPI-gSlider acquisition.$$$\,B_0\,$$$is used to perform post-processing distortion correction to un-distort EPI.
Results.
Acceleration Experiments: Figure 3 presents results from a selected under-sampled case that achieves good trade-off with respect to speed versus recovered map quality. This constitutes an 11 second PhysiCal scan at$$$\,48\times\,$$$acceleration across$$$\,12\,$$$echoes. Difference maps with respect to gold standard demonstrate high quality of reconstruction that accurately captures high frequency spatial variations in$$$\,B_0\,$$$and$$$\,B_1^+\,$$$maps. Non-linear eddy current fields between odd-even echoes is also captured that could prove useful in improving EPI ghost-correction (at matched echo spacing) over standard 1D ghost-correction.Preliminary Efficacy Verification: Results are depicted in Figure 4.$$$\,B_1^+\,$$$obtained from the 11 second accelerated case is successful at mitigating striping artifacts in EPI-gSlider.$$$\,B_0\,$$$is successful at performing post-processing distortion correction to match the EPI image to a distortion-free structural image. However, it cannot resolve signal in voxel pile-up areas, which would require more data (for example, from a 2-shots blip-up and blip-down EPI).
Discussion and Future Work.
A rapid multi-parametric calibration scan termed PhysiCal is proposed and demonstrated to capture accurate high-resolution whole-brain$$$\,B_1^+$$$, CSM,$$$\,B_0\,$$$and eddy current maps in 11 seconds. This rapid acquisition is achieved through tailored under-sampling and synergistic constrained+eigenvalue reconstruction. Future work includes prospective under-sampling implementation and evaluation at ultra-high field with along with utility in pTx $$$B_1^+$$$ calibration. It is expected that the increased $$$B_1^+$$$ variations in these applications will still be captured well by the eigenvalue approach given that ESPRiT successfully estimates $$$B_1^-$$$ for local coil arrays. More advanced reconstruction approaches through low-rank and phase-constraints will also be explored to aid an even faster and robust calibration scan. Additionally, the efficacy of PhysiCal will be verified on other applications including spatio-temporal encoding like EPTI12 and MR fingerprinting13.Acknowledgements
This work was supported in part by NIH research grants: NIH R01EB019437, R01EB020613, R01 MH116173, and U01EB025162.References
- Uecker, Martin, Peng Lai, Mark J. Murphy, Patrick Virtue, Michael Elad, John M. Pauly, Shreyas S. Vasanawala, and Michael Lustig. "ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA." Magnetic resonance in medicine 71, no. 3 (2014): 990-1001.
- Ying, Leslie, and Jinhua Sheng. "Joint image reconstruction and sensitivity estimation in SENSE (JSENSE)." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 57, no. 6 (2007): 1196-1202
- Polimeni, Jonathan R., Himanshu Bhat, Thomas Witzel, Thomas Benner, Thorsten Feiweier, Souheil J. Inati, Ville Renvall, Keith Heberlein, and Lawrence L. Wald. "Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition." Magnetic resonance in medicine 75, no. 2 (2016): 665-679.
- Zahneisen, Benjamin, Murat Aksoy, Julian Maclaren, Christian Wuerslin, and Roland Bammer. "Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction." NeuroImage 153 (2017): 97-108.
- Sacolick, Laura I., Florian Wiesinger, Ileana Hancu, and Mika W. Vogel. "B1 mapping by Bloch‐Siegert shift." Magnetic resonance in medicine 63, no. 5 (2010): 1315-1322.
- Lesch, Andreas, Matthias Schlöegl, Martin Holler, Kristian Bredies, and Rudolf Stollberger. "Ultrafast 3D Bloch–Siegert B‐mapping using variational modeling." Magnetic resonance in medicine 81, no. 2 (2019): 881-892.
- Corbin, Nadege, Julio Acosta-Cabronero, Shaihan J. Malik, and Martina F. Callaghan. "Robust 3D Bloch-Siegert based B1+ mapping using Multi-Echo General Linear Modelling." bioRxiv (2019): 550509.
- Setsompop, Kawin, Qiuyun Fan, Jason Stockmann, Berkin Bilgic, Susie Huang, Stephen F. Cauley, Aapo Nummenmaa et al. "High‐resolution in vivo diffusion imaging of the human brain with generalized slice dithered enhanced resolution: Simultaneous multislice (gSlider‐SMS)." Magnetic resonance in medicine 79, no. 1 (2018): 141-151.
- Liao, Congyu, Jason Stockmann, Qiyuan Tian, Berkin Bilgic, Nicolas S. Arango, Mary Kate Manhard, Susie Y. Huang, William A. Grissom, Lawrence L. Wald, and Kawin Setsompop. "High‐fidelity, high‐isotropic‐resolution diffusion imaging through gSlider acquisition with and T1 corrections and integrated ΔB0/Rx shim array." Magnetic resonance in medicine 83, no. 1 (2019): 56-67.
- Lesch, Andreas Johann, Andreas Petrovic, and Rudolf Stollberger. "Robust implementation of 3D Bloch Siegert B1 mapping." In Proc. Intl. Soc. Mag. Reson. Med. 23. ., 2015.
- Uecker, Martin, Frank Ong, Jonathan I. Tamir, Dara Bahri, Patrick Virtue, Joseph Y. Cheng, Tao Zhang, and Michael Lustig. "Berkeley advanced reconstruction toolbox." In Proc. Intl. Soc. Mag. Reson. Med, vol. 23, no. 2486. 2015.
- Wang, Fuyixue, Zijing Dong, Timothy G. Reese, Berkin Bilgic, Mary Katherine Manhard, Jingyuan Chen, Jonathan R. Polimeni, Lawrence L. Wald, and Kawin Setsompop. "Echo planar time‐resolved imaging (EPTI)." Magnetic resonance in medicine 81, no. 6 (2019): 3599-3615.
- Ma, Dan, Vikas Gulani, Nicole Seiberlich, Kecheng Liu, Jeffrey L. Sunshine, Jeffrey L. Duerk, and Mark A. Griswold. "Magnetic resonance fingerprinting." Nature 495, no. 7440 (2013): 187.
Figures
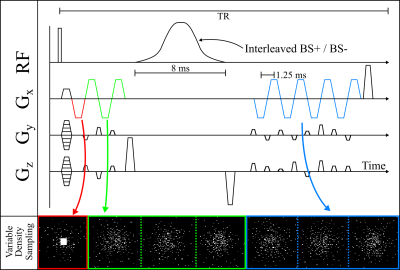
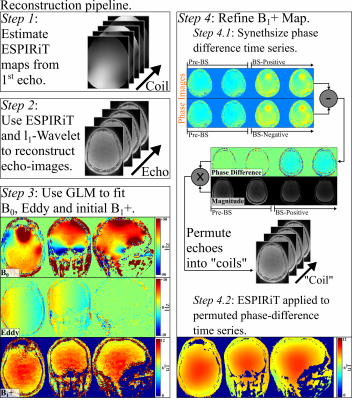
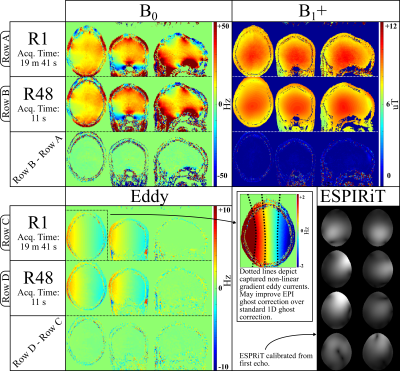
Fig 3: This figure compares the accelerated parametric maps (R48) to the gold standard (R1). By designing tailored under-sampling and using synergistic constrained+eigenvalue reconstruction, accurate and high resolution $$$B_0$$$, Eddy, $$$B_1^+$$$ and coil sensitivity maps can be recovered in 11 seconds.