0660
Respiration-Resolved 3D Multi-Channel B1 mapping of the body at 7T1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
A major challenge of ultra-high field MRI is the spatially inhomogeneous transmit radio frequency field that induces spatial contrast variations. In this work we present a novel technique to retrieve channel-wise, motion-resolved absolute 3D FA maps of the human body at 7T. Free breathing 3D scans of the thorax were performed and B1 maps without motion artifacts are obtained for the two motion states inhale and exhale. It allows acquiring B1 libraries that can be used for offline RF pulse calculation including motion-robust RF pulses.
Introduction
A major challenge of ultra-high field MRI is the spatially inhomogeneous transmit (TX) radio frequency field ($$$B_1^+$$$) that induces spatial contrast variations. This problem has been addressed by combinations of multi-channel TX coils, spatial mapping of$$$\,B_1^+\,$$$or the flip angle (FA), and by $$$B_1$$$ shimming or parallel transmission1,2. Mapping channel wise independent$$$\,B_1^+\,$$$maps over a 3D volume covering the human brain has been achieved by combining a single, absolute$$$\,B_1^+\,$$$map (e.g. acquired by the actual flip angle (AFI) technique3) with multiple relative maps4, or directly by other approaches such as DREAM5. In contrast, 3D mapping of$$$\,B_1^+\,$$$fields in the body is challenging, in particular due to respiration and specific absorption rate (SAR) limitations. Thus, several UHF body studies targeting the heart, aorta and other organs6,7,8 have successfully applied a fast (breathold-long) and low power 2D technique, that derives proton-density biased, complex$$$\,B_1^+\,$$$maps from multiple gradient echo scans. The resulting relative$$$\,B_1^+\,$$$maps essentially miss a spatially constant scaling factor λ between such relative and absolute maps. Despite these limitations, the technique has proven to be highly suitable, even for parallel transmission in cardiac MRI7. The present work shows a novel, UHF$$$\,B_1^+\,$$$mapping technique that has three features: First, it extends the aforementioned method towards 3D$$$\,B_1^+\,$$$mapping for UHF body MRI; second it determines the scaling factor λ to obtain absolute B1+ maps and third, since the scan runs under free-breathing it yields multiple 3D $$$B_1^+$$$ maps of different respiratory states.Methods
First,$$$\,K\times R\,$$$three-dimensional respiration-resolved relative $$$B_1^+$$$ maps $$$\,\widetilde{B_{1,k,r}^{rel}}\,$$$for each channel $$$k=1\ldots K$$$ and respiratory state $$$r=1\ldots R$$$ were derived using a technique similar to9. The tilde denotes, that maps are estimated/biased. To this end, a radial phase-encoded (RPE)10,11 3D gradient-echo acquisition (RPE-GRE,cf. fig.1) was acquired$$$\,K\,$$$times and for each acquisition only one TX channel$$$\,k\,$$$transmitted while all others were inactive. The$$$\,K\times R=8\times10\,$$$gradient-echo (GRE) images$$$\,I_k\,$$$were reconstructed using self-navigation, retrospective respiratory binning (sliding window with 40% overlap) and NUFFT based on a k-t SENSE algorithm11,12,13. Subsequently, individual maps $$ \widetilde{B_{1,k,r}^{rel}} = \frac{I_{k,r}}{(M_{z0}\sum_k\lvert I_{k,r}\rvert)^{\frac{1}{2}}} $$were derived similar to9 with $$$M_{z0}$$$ proportional to proton density. Subsequently an RPE-based AFI3,14 was acquired using a circular polarized (CP) mode$$$\,B_1^+$$$-shim yielding respiration-resolved absolute$$$\,B_1^+$$$ maps$$$\,B_{1,r}^{CP}$$$, which, however, exhibited local regions with low FA (<10degrees) that are excluded from further post processing. Such maps were merged with gradient-echo images resulting in absolute maps (with undefined regions) of channel$$$\,k$$$ and respiratory state$$$\,r$$$: $$$\widetilde{B_{1,k,r}^{abs}}=\frac{\lvert{I_{k,r}\rvert}}{\lvert{\sum_1^KI_{k,r}}\rvert}B_{1,r}^{CP}$$$ 4. Note, that$$$\,\lvert{\sum_1^KI_{k,r}}\rvert$$$ generates an image transmitted in CP mode. Finally the scaling factor $$$λ$$$ is calculated based on an ROI $$$\Gamma$$$ with high$$$\,B_1^+\,$$$amplitude revealing estimated, but absolute $$$B_1^+$$$ maps:$$$\widetilde{B_{1,k,r}^{abs}}=\lambda\cdot\widetilde{B_{1,k,r}^{rel}} $$$ with $$$\lambda = \frac{\widetilde{B_{1,k,r}^{abs}}}{\widetilde{B_{1,k,r}^{rel}}}\Bigg\rvert_{\Gamma}$$$.
All scans were performed at 7T (Magnetom 7T, Siemens, Germany) and in-vivo scans according to an approved IRB protocol. An initial phantom scan was performed using an 8-channel transceiver head coil using an agarose-water phantom ($$$σ=0.4\text{s}/\text{m},\, ε=79$$$) which performed a periodic ($$$f=0.15\text{Hz}$$$) translational movement in bore direction. The technique was evaluated at both extremal positions and resulting maps were compared to identical acquisitions with the phantom being static at those two positions. Five subjects were scanned using an 8-channel transceiver body coil. All reconstructed$$$\,B_1^+\,$$$maps were converted to FA maps for a reference voltage of$$$\,U_{ref} = 30\text{V}/\,160\text{V}\,$$$(phantom/in-vivo) and nominal FA = 60°/32°. Acquisition times of the RPE-GRE and RPE-AFI were 10.25 and 12.25min, reconstruction time was approximately 30min.
Results
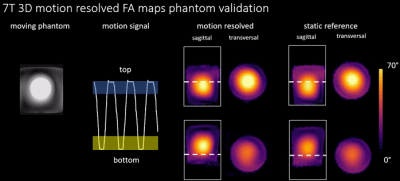
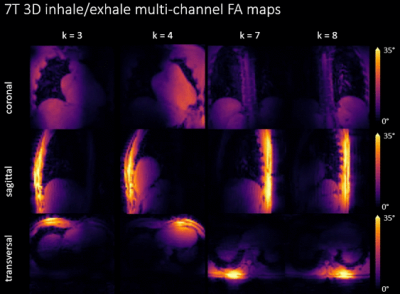
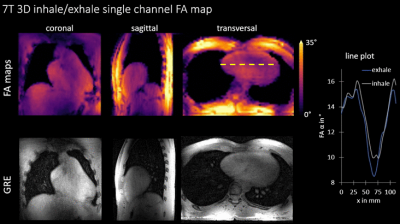
Fig.2 shows the derived phantom navigator signal and compares motion resolved 3D FA maps of two locations to a static reference measurement at same phantom locations. Quantitatively, the mean difference between static reference and dynamic measurement is$$$\,\overline{\Delta\alpha}=(0.8±12)°\,$$$(bottom) and$$$\,\overline{\Delta\alpha}=(-1.6±8)°\,$$$(top). Respiration-resolved in-vivo 3D FA maps from four out of the eight TX channels in one of the five subjects are illustrated in Fig.3. As can be seen, clean maps free from motion artifacts are obtained for the two motion states inhale and exhale. Fig.4, bottom displays RPE-GRE images obtained after homogeneous$$$\,B_1^+\,$$$shimming targeting the heart, which was performed in a single slice using an existing 2D mapping method as reported in7. The same shim set was retrospectively applied to the motion-resolved channel-wise FA data and the resulting FA maps after combining all TX-channels (Fig.4 top) which qualitatively agree with the obtained GRE images. Cross-sections of the FA distribution through the heart (dashed yellow line) for exhale and inhale demonstrate FA variations of up to 10% between both respiratory states, which agrees with previous work15.Dicussion & Conclusion
In this work we present a novel technique to retrieve channel-wise, motion-resolved absolute 3D$$$\,B_1^+\,$$$maps of the human body at 7T. Although the maps inherently contain a proton density bias, similar non-respiration-resolved maps obtained in 2D have been successfully for cardiac PTX and other applications at 7T. Ideally, a direct merging of RPE-AFI and GRE-AFI is preferable, which was unfeasible because of insufficient B1+ amplitudes were present in the center of the body. The present method circumvents this limitation by using AFI data only in regions with high$$$\,B_1^+\,$$$amplitude for calibration purposes. Note, that the proposed method is not intended for online RF-pulse design, which is prevented by long acquisition and reconstruction times. Instead it allows acquiring libraries that can be used for offline RF pulse calculation16 including motion-robust RF pulses15.Acknowledgements
Support of the German Research Foundation (DFG), project number GRK 2260, BIOQIC is acknowledged.References
1. Kerr, Etezadi-Amoli, Fautz, Vogel, Gross, Zhu, Pauly. Proc ISMRM 16, (2008)
2. Padormo, Beqiri, Hajnal, Malik. NMR in Biomedicine 29,1145-1161 (2016)
3. Yarnykh. Magentic Resonance in Medicine 57, 192-200 (2007)
4. Van de Moortele, Snyder, DelaBarre, Adriany, Vaughan, Uğurbil. Proc. ISMRM: 1676 (2007)
5. Nehrke, Versluis, Webb, Börnert. Magnetic Resonance in Medicine 71: 246-256 (2014)
6. Metzger, Auerbach, Akgun, Simonson, Bi, Uğurbil, Van de Moortele. Magnetic Resonance in Medicine 69: 114-126 (2013)
7. Schmitter, DelaBarre, Wu, Greiser, Wang, Auerbach, Vaughan, Uğurbil, Van de Moortele. Magnetic Resonance in Medicine 70: 1210-1219 (2013)
8. Hess, Bissell, Ntusi, Lewis, Tunnicliffe, Greiser, Stalder, Francis, Meyerson, Neubauer, Robson. Magnetic Resonance in Medicine 73: 1864-1871 (2015)
9. Van de Moortele, Ugurbil. Proc. ISMRM: 367 (2009)
10. Prieto, Uribe, Razavi, Atkinson, Schaeffter. Magnetic Resonance in Medicine 68, 514-526 (2010)
11. Buerger, Clough, King, Schaeffter, Prieto. Trans Med Imaging 31, 805-815 (2012)
12. Jackson, Meyer, Nishimura, Macovski. IEEE Transactions on Medical Imaging 10: 473–478 (1991)
13. Tsao, Boesiger, Pruessmann. Magnetic Resonance in Medicine 50: 1031-1042 (2003)
14. Dietrich, Kolbitsch, Schaeffter, Schmitter. Proc. ISMRM: 4560 (2019)
15. Schmitter, Wu, Uğurbil, Van de Moortele. Magnetic Resonance in Medicine 74: 1291-1305 (2015)
16. Gras, Vignaud, Amadon, Bihan, Boulant. Magnetic Resonance in Medicine, 77: 635-643 (2017)
Figures