0656
Concurrent Field Monitoring in HCP dMRI at 7T: Correction for Eddy Current Induced Signal Blurring and Geometric Distortion.
Ruoyun Emily Ma1, Mehmet Akçakaya1,2, Steen Moeller1, Connor Benson1, Edward Auerbach1, Kâmil Uğurbil1, and Pierre-François Van de Moortele1
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States
Synopsis
DW-EPI at high diffusion gradients suffers from eddy current induced signal blurring and geometric distortion. In this study, concurrent monitoring of field evolution with NMR probes was implemented for HCP diffusion MRI acquisition at 7T. After image reconstruction with field correction, eddy current induced geometric distortion was largely removed, yielding similar level of correction obtained by data driven approaches such as EDDY. Signal blurring was further reduced than with EDDY. Future efforts will be made to quantify the impact on cortical tractography of this improved DW-EPI reconstruction.
Introduction
Recent development in field monitoring powered by NMR field probes enables directly obtaining actual field information during MR signal encoding, providing a way to retrospectively correct for field perturbations caused by hardware imperfection and physiological noise1–3. In this study, we applied this approach to monitor field evolution simultaneously with in vivo diffusion MRI acquisitions at 7T following human connectome project (HCP) protocols4, aiming at correcting for eddy current (EC) induced signal blurring and geometric distortion. The effectiveness of field correction was compared to the commonly used data-driven approach EDDY5.Methods
dMRI acquisition:All acquisitions were conducted on a 7T whole body Siemens scanner (Siemens Healthineers, Erlangen, Germany) with a 1x32 channel head coil (Nova Medical, Wilmington, MA, USA). Two in vivo acquisitions were performed. Diffusion weighted (DW) images were acquired using the multi-band (MB) EPI sequence as described in4. Key parameters include: two shells in q-space sampling (b=1000 and 2000s/mm2, containing 64 diffusion gradients for each shell, and 15 b=0 volumes), TR/TE=7000ms/71.2ms, imaging resolution=(1.05mm)3, MB=2 with blipped-CAIPI6, in-plane acceleration=3, 66 slices, 1 average.
Concurrent field monitoring:
16 19F NMR probes (Skope, Zuirch, CH) were placed in between the transmitter and receiver coils. The signal reading of NMR probes started right prior to the phase encoding gradient of each EPI readout. The 0th, 1st and 2nd field terms were fitted by the field acquisition system. The standard deviation of each term, calculated7 assuming an SNR of 10, is listed in Table 1. The field monitoring was conducted concurrently with the in vivo dMRI acquisition. A separate measurement with the same setup of the coil and the probes was performed on a phantom to obtain field evolution without physiological noise. Thus, field fluctuations caused by physiological activities can be extracted by calculating the difference between the fields measured in phantom and in vivo acquisitions. The field deviation induced by EC was obtained from the phantom acquisition by subtracting the field measured during the first EPI with b-value of 0 from all other EPI readouts.
Image reconstruction and post-processing:
The pipeline of image reconstruction with field correction and post-processing to obtain diffusion parametric maps is shown in Fig. 1. Complex DW MR images were reconstructed using an algebraic CG-SENSE approach8 after removing scanner imposed 0th order EC compensation and off-center phase compensation for CAIPI acquisition9. 2D denoising was performed using MP-PCA10 with the window size of 11x11. To account for rigid head movement across diffusion encoding steps, volume-to-volume registration was performed with the coreg-reslice function in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) for each bvalue separately.
To examine the effect of EC correction using field monitoring, single-shot SMS SENSE reconstruction11 was also performed followed by three types of post-processing strategies:
i) FSL-EDDY5 processing to address both EC induced artifacts and rigid head movement across volumes; ii) Volume-to-volume registration with SPM12 to only correct rigid head movement; this approach is used to distinguish the effect of head movement and geometric incongruence induced by EC.
iii) No further processing.
Colored fractional anisotropy (cFA) maps were generated using MRtrix312. To focus on the artifacts from EC, only the data acquired with the phase encoding direction of AP was included in the analysis.
Results
Field monitoring for EC and physiological noise:Fig.2 shows the physiological noise and EC induced field perturbation for all measured field terms during 84.8s. The periodic fluctuation of the field induced by breathing can be well appreciated. Comparing the left and right columns of Fig.2, it could be seen that the field deviation in this particular acquisition was mainly contributed by EC.
EC correction on DWI:
The comparison of reconstructed images using the four aforementioned processing approaches is displayed in Fig.3. As shown in the top row with a DW image with bvalue of 2000, the blurring in the anterior frontal area is successfully corrected with field monitoring, with slightly better performance than EDDY. The effect of EC correction on reducing the geometric incongruence is displayed in the second row, showing the mean DWI averaged from all 64 DW images with bvalue of 2000. The delineation between gray and white matter is better revealed after field correction or EDDY processing. The parametric maps obtained from images with field correction and EDDY processing yield similar results. As shown in the third row, both strategies result in cleaner cFA maps compared to the cases where no EC correction was applied, especially in the frontal area.
Discussion and conclusion
In this study, the effective EC correction was achieved with concurrent field monitoring and field correction on HCP diffusion MRI acquisition. Compared with EDDY, the field correction based reconstruction provides better results with reduced blurring in the final images. When pushing spatial resolution at ultrahigh field at the limits of affordable SNR, this improved point spread function obtained when using field monitoring is expected to improve the quality and fidelity of final parametric reconstruction,such as in tractography particularly in cortical areas. Current efforts are made towards this direction.Acknowledgements
This work received financial support from the National Institutes of Health (NIH): P41 EB015894; P30 NS076408; P41 EB027061; U01 EB025144; and from the National Science Foundation (NSF): Award 1607835References
- Vannesjo SJ, Wilm BJ, Duerst Y, et al. Retrospective correction of physiological field fluctuations in high-field brain MRI using concurrent field monitoring. Magn Reson Med. 2015;73(5):1833-1843. doi:10.1002/mrm.25303
- Dietrich BE, Brunner DO, Wilm BJ, et al. A field camera for MR sequence monitoring and system analysis. Magn Reson Med. 2016;75(4):1831-1840. doi:10.1002/mrm.25770
- Wilm BJ, Nagy Z, Barmet C, et al. Diffusion MRI with concurrent magnetic field monitoring. Magn Reson Med. 2015;74(4):925-933. doi:10.1002/mrm.25827
- Vu AT, Auerbach E, Lenglet C, et al. High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. Neuroimage. 2015;122:318-331. doi:10.1016/j.neuroimage.2015.08.004
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-1078. doi:10.1016/j.neuroimage.2015.10.019
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. doi:10.1002/mrm.23097
- Barmet C, De Zanche N, Pruessmann KP. Spatiotemporal magnetic field monitoring for MR. Magn Reson Med. 2008;60(1):187-197. doi:10.1002/mrm.21603
- Wilm BJ, Barmet C, Pavan M, Pruessmann KP. Higher order reconstruction for MRI in the presence of spatiotemporal field perturbations. Magn Reson Med. 2011;65(6):1690-1701. doi:10.1002/mrm.22767
- Ma RE, Akçakaya M, Moeller S, Auerbach EJ, Uǧurbil K, Van de Moortele PF. Correcting Eddy Current Induced Geometric Distortion for High Resolution Multi-Band Diffusion Weighted SE-EPI with Magnetic Field Monitoring at 7T. Proc Intl Soc Mag Reson Med. 2019;27:0922.
- Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406. doi:10.1016/j.neuroimage.2016.08.016
- Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006;55(3):549-556. doi:10.1002/mrm.20787
- Tournier J-D, Smith R, Raffelt D, et al.
MRtrix3: A fast, flexible and open software framework for medical image
processing and visualisation. Neuroimage. 2019;202(August):116137.
doi:10.1016/j.neuroimage.2019.116137
Figures
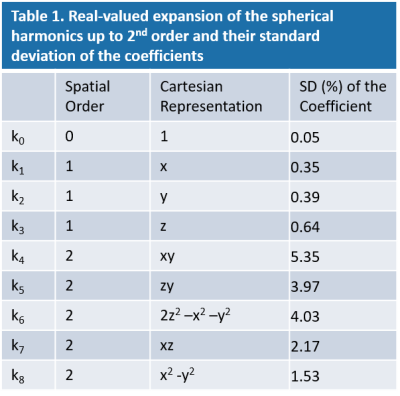
Table
1. Real-valued expansion of the spherical harmonics up to 2nd
order and their standard deviation of the coefficients
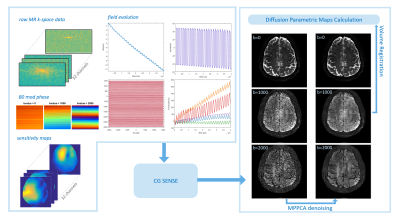
Fig.1 Pipeline of image reconstruction
and post processing for field correction
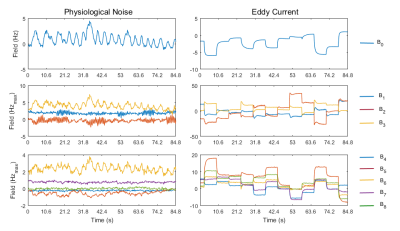
Fig.2. field fluctuations in the case of
physiological noise (left column) and eddy current (right column). Fields
fitted from 0th, 1st and
2nd order spherical harmonics (Table 1) are
displayed. All the field terms are scaled to maximum field shift within a
sphere of 10cm radius (Hzmax).
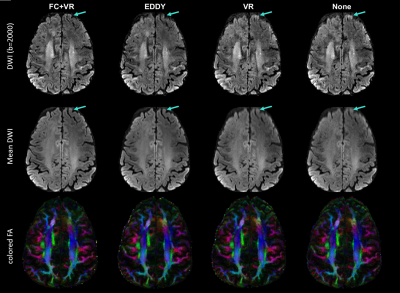
Fig.3. Comparison of DW images (top row),
mean DW images (middle row) and cFA maps (bottom row) obtained from images
reconstructed/processed with field correction + volume registration (FC+VR),
FSL-EDDY, VR only and no correction (None). FSL-EDDY, VR only and None were
operated on images with single-shot SENSE reconstruction.