0649
MRI Detection of Impairment of Glymphatic Function in Rat after Mild Traumatic Brain Injury
Lian Li1, Michael Chopp1,2, Guangliang Ding1, Esmaeil Davoodi-Bojd1, Qingjiang Li1, Yanlu Zhang1, Ye Xiong1, and Quan Jiang1
1Henry Ford Hospital, Detroit, MI, United States, 2Oakland University, Rochester, MI, United States
1Henry Ford Hospital, Detroit, MI, United States, 2Oakland University, Rochester, MI, United States
Synopsis
Using dynamic MRI glymphatic measurement and our advanced mathematic model, the alterations of glymphatic function in the brain with mild TBI were investigated. Our data show that mild TBI leads to both impaired influx and efflux of contrast agent along the glymphatic pathway. The reduced efficiency of glymphatic function affects the multiple regions across the brain, which may decrease the clearance of waste metabolites and facilitate protein aggregation, contributing to subsequent cognitive deficits. The global change in brain clearance function, rather than the appearance of focal lesions, appears to provide a reliable measure indicating the injury of the brain.
Introduction
As a brain-wide perivascular network, the glymphatic system allows cerebrospinal fluid (CSF) to exchange with interstitial fluid (ISF), facilitating the clearance of waste from the brain1. Impairment of glymphatic system is found in many neurological diseases, including traumatic brain injury (TBI), and is linked to associated long-term cognitive deficits. However, most of previous studies mainly used two-photon and histological methods to study the changes of glymphatic system resulting from TBI1-2. There is a lack of whole brain investigation, especially conducted in vivo, on the alterations of glymphatic function after mild TBI, that accounts for at least 75% of brain injuries. In addition, definitive diagnosis of mild TBI with routine neuroimaging modalities remains a challenge3. The changes in brain function, such as the variance in glymphatic clearance that plays an important role in the trauma-induced cognitive sequelae, may offer a sensitive and confirmatory assessment for this neurological injury. For mathematical modeling of the glymphatic activity, we recently developed an advanced two-compartment kinetic model4. Instead of the global input function from the injection site, we used the local input function obtained from clustering the tissues based on their dynamic responses to the infusion of contrast agent. By fitting the model to the local cerebral regions rather than to the averaged time signal curve (TSC) of the whole brain, more accurate kinetic parameters that characterize the specific features for the dynamic transport of contrast agent in a living brain were then derived. With this advanced model and derived parameters, we perform the present study, for the first time, in the field of mild TBI. By calculating parametric maps and quantifying in typical tissue regions across the brain, the present investigation was designed to reveal whether the impairment of glymphatic pathway function occurred as a result of mild TBI and the manner in which the glymphatic function was altered in the injured brain.Methods
Mild TBI was induced by a closed head impact5. Male Wistar rats with and without TBI (~400g, n=7/group) underwent the identical MRI protocol 10-weeks post-injury. T2WIs (TE=15, 30, 45, 60, 75, and 90ms, TR=4.5s, FOV=32x32mm2, matrix=128x128, 13 slices, thickness=1mm) were measured for detection of brain tissue changes. To monitor the dynamic influx and clean-out process, 3D T1WIs (TE=4ms, TR=15ms, flip angle=15°, FOV=32x32x16mm3, and matrix=256×192×96) with contrast agent of Gd-DTPA was employed. The acquisition of T1WIs continued for 6h with initial three baseline scans followed by intra-cisterna magna Gd-DTPA (21mM concentration) delivery via the indwelling catheter at an infusion rate of 1.6ml/min over 50min6. Based on the TSC in each cluster, parameters of infusion rate and clearance rate representing the slope of signal increase in the accumulation phase and the slope of signal decrease in the relaxing phase of the TSC, respectively, were calculated. The clearance time constant was obtained by fitting a one exponential model to the relaxing phase of the TSC. Parametric maps of infusion rate, clearance rate and clearance time constant for whole brain were then generated, and quantitative evaluation on these maps in the regions of interest (ROIs, Fig. 1) was performed.Results
There was no lesion or injury present on conventional structural images, such as T1WIs and T2WIs. The dynamic profiles for both influx and clean-out process of contrast agent, however, demonstrated the brain alteration in glymphatic function as a result of mild TBI. In all measured ROIs that encompassed major typical tissue areas in the brain, lower infusion rate (Fig. 2A) and clearance rate (Fig. 2B) were found for the TBI animals compared to the healthy controls, with differences in the majority of regions reaching statistical significance. In addition, significantly higher clearance time constant values (Fig. 2C) in the most measured regions were detected in the TBI group than in the control group.Discussion and Conclusion
Even without visible lesions, the alterations of glymphatic function in the brain with mild TBI, as monitored by dynamic MRI glymphatic measurement and characterized by parametric maps, can be revealed using our advanced mathematic model4. The change in brain clearance function, rather than the appearance of focal lesions, appears to provide a reliable measure indicating the injury of the brain, particularly at the setting of mild TBI, and should be therefore considered as one of important factors leading to the neurobehavioral sequelae. Our data show that mild TBI markedly insults both influx and efflux of contrast agent along the glymphatic pathway. The reduced efficiency of glymphatic function affects the multiple regions across the brain rather than within the restricted areas associated to the impact site. The globally impaired glymphatic function may decrease the clearance of waste metabolites and facilitate protein aggregation1, which in turn contribute to neurodegeneration and subsequent cognitive deficits5.Acknowledgements
This work was supported by NIH grants RO1 NS064134 (Jiang), R21AG052735 (Jiang), NS079612 (ZG Zhang), NS062832 (L Zhang), AG037506 (Chopp).References
- Iliff JJ, Chen MJ, Plog BA, et al. Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. J Neurosci. 2014; 34(49): 16180-93.
- Plog BA, Dashnaw ML, Hitomi E, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015; 35(2) :518-26.
- Shetty T, Nguyen JT, Cogsil T, et al. Clinical Findings in a Multicenter MRI Study of Mild TBI. Front Neurol. 2018; 9: 1-10.
- Davoodi-Bojd E, Ding G, Zhang L, et al. Modeling glymphatic system of the brain using MRI. NeuroImage 2019; 188: 616-627.
- Zhang Y, Chopp M, Meng Y, et al. Cerebrolysin improves cognitive performance in rats after mild traumatic brain injury. 2015; J Neurosurg 122: 843–855.
- Jiang Q, Zhang L, Ding G, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017; 37 (4): 1326-1337.
Figures
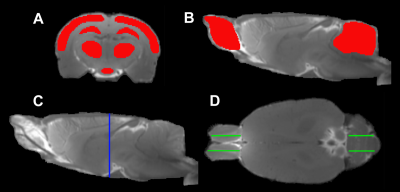
Fig. 1 ROIs
(red in A, B) on the coronal (A)
and sagittal (B) sections, and the
corresponding locations seeing on the sagittal (C, blue line) and axial (D,
green lines) sections, respectively. The symmetric ROIs in the same tissue
structure (D, green lines in OB
and cerebellum) provide average measurements.
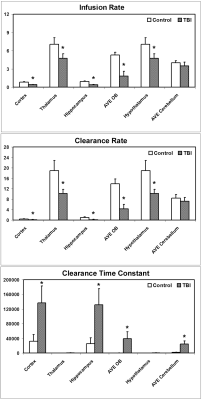
Fig. 2 Infusion rate (A),
clearance rate (B) and clearance time constant (C) in different
regions for rats with and without TBI.