0647
Human meningeal lymphatic vessels can be imaged by inversion recovery alternate ascending/descending directional navigation (ALADDIN)1Department of Bio and Brain engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of
Synopsis
Recent studies showed meningeal lymphatic vessels significantly contribute to the clearance mechanisms of cerebrospinal fluid (CSF) and the immune system in central nervous system. In this study, we tried to image human dural meningeal lymphatic vessels (mLVs) using inversion recovery alternate ascending/descending directional navigation (IR-ALADDIN). The IR-ALADDIN imaging technique clearly showed not only structural dural mLVs, but also the flow direction of dural mLVs, and it can be applied for studying many lymphatic vessels in human neurological diseases.
Introduction
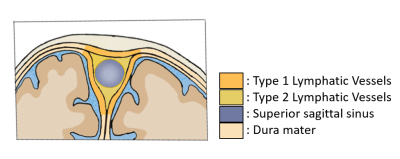
Meningeal lymphatic vessels play an important role in the clearance mechanisms of cerebrospinal fluid(CSF) and the immune system in central nervous system [1-3]. The dural meningeal lymphatic vessels(dural mLVs), which are mainly distributed around superior sagittal sinus(SSS), are closely related with glymphatic system(Fig.1). The glymphatic system clears soluble proteins and waste products from CSF by interaction with the meningeal lymphatic vessels [4, 5]. Imaging human dural mLVs is an important research topic in this regard. So far, most of human dural mLVs imaging methods involve intravenous or intrathecal injection of contrast agent [4, 6], and noninvasive imaging methods are rare [7], because of the small size and the slow flow in dural mLVs.Alternate ascending/descending directional navigation(ALADDIN) is a noninvasive arterial spin labeling imaging technique where labeling planes automatically track the imaging plane with no separate labeling/control scan[8]. Imaging with higher spatial resolution is advantageous for ALADDIN in that the prior slices work as labeling planes for the subsequent slices for a longer time. These unique features of ALADDIN may be advantageous for imaging dural mLVs. In this research, we used inversion recovery ALADDIN(IR-ALADDIN) [8]for imaging dural mLVs without contrast agent.
Method
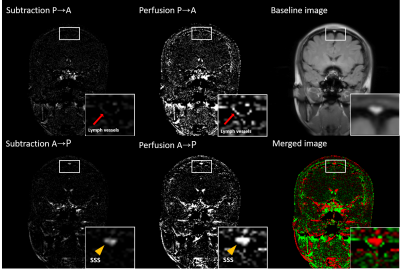
All experiments were performed on a 3T whole-body scanner(Siemens Medical Solutions) with a body-coil transmission and a head coil reception. Total 4 normal volunteers were scanned in this study approved by the Institutional Review Board. To get dural mLVs images, inversion recovery 2D bSSFP imaging technique(ALADDIN) was used. The ALADDIN imaging were performed in the ascending and descending orders with positive/negative slice-selection gradients and positive/negative readout gradients in an alternating manner, yielding total 8 different measurements that composed one set. This imaging with alternating orders and gradients compensates for MT asymmetry, eddy current artifact, gradient imperfection and field inhomogeneity [9]. Inversion recovery pulse with inversion time 2300ms was applied for each slice in order to suppress CSF adjacent to SSS. Imaging parameters were TR/TE =4.07/2msec, flip angle =60°, matrix size =256×256, field of view =250×250 mm2, thickness =5 mm, gap =6 mm (120% of thickness), scan direction =coronal, PE order =linear, and PE direction = left–right. Two full sets were acquired with number of slices = 19 and then another two full sets were acquired with number of slices = 18. The former and the latter covered the whole brain at a conventional gap value of 0.5 mm with total scan time = 7min 24sec. Four ascending acquisitions and four descending acquisitions were averaged separately and then subtraction was performed between the two averaged acquisitions to maximize the flow signals which have directionality. To visualize the lymphatic vessels better, the images were displayed as subtraction images (Asc-Dsc) as well as percent signal changes(PSC) (Asc-Dsc)/S*100, where Asc and Dsc represent the averaged ascending and descending images and S represents average of Asc and Dsc.Results
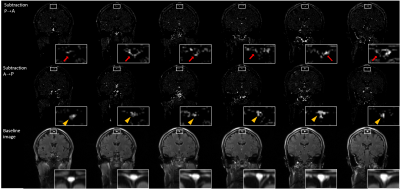
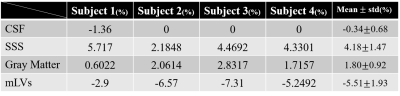
The three different processing schemes of IR-ALADDIN images showed high flow pixels in SSS in the direction of anterior to posterior(A->P), whereas high flow pixels were found in the periphery of SSS in the direction of posterior to anterior(P->A) where dural mLVs are mainly distributed(Fig.2). Dural mLVs appeared through multiple slices in one subject(Fig.3), and also it was shown in multiple subjects(Fig.4). The PSC values of CSF, SSS, gray matter, dural mLVs were calculated from ALADDIN perfusion images(Fig.5). Each anatomical region had different PSC distributions, supporting the notion that the dual mLVs signals are not from CSF, SSS, and brain tissue(GM).Discussion and Conclusion
We postulate the high flow signals around SSS are about lymphatic vessels. First, we can assume that they are not from CSF, because we used IR-ALADDIN with inversion time 2300ms. T1 of lymphatic vessels(3100 msec) is different from that of CSF(4163 msec). Second, the high signals around SSS showed flow direction of posterior to anterior, which was opposite to the flow direction of SSS and in agreement with the known flow direction of dural mLVs[7]. Third, in ALADDIN imaging scheme, prior imaging slices act as labeling slices to the subsequent imaging slices and the gap between 2 slices was 6mm in this study. So even the slow lymphatic flow could be detected. Finally, based on the previous morphology studies, there are tubular shaped lymphatic vessels in the periphery of SSS and some lymphatic vessels were distributed around the SSS through the dura mater. These morphological shape and distribution of dural mLVs from the previous studies[4, 6], coincide with those of the high flow signals around SSS in this study. PSC distributions of dural mLVs were completely different from those of CSF, SSS, and gray mater(Fig.5). Based on all these factors, we believe that the high flow signals around SSS in this study are about dural mLVs.IR-ALADDIN imaging technique precisely demonstrated not only structural dural mLVs, but also the flow direction of dural mLVs without using contrast agent. Because of the noninvasiveness, high resolution, sensitivity to the slow flow, and availability of the flow directional information, the proposed IR‑ALAADIN approach can be applied for studying many lymphatic vessels in human neurological diseases such as Alzheimer’s disease and multiple sclerosis.
Acknowledgements
No acknowledgement found.References
1. Louveau, A., et al., Structural and functional features of central nervous system lymphatic vessels. Nature, 2015. 523(7560): p. 337-41.
2. Aspelund, A., et al., A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med, 2015. 212(7): p. 991-9.
3. Ahn, J.H., et al., Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature, 2019. 572(7767): p. 62-66.
4. Absinta, M., et al., Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife, 2017. 6.
5. Bacyinski, A., et al., The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front Neuroanat, 2017. 11: p. 101.
6. Goodman, J.R., et al., Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer's dementia subjects. Brain Behav Immun, 2018. 73: p. 34-40.
7. Kuo, P.H., et al., Meningeal Lymphatic Vessel Flow Runs Countercurrent to Venous Flow in the Superior Sagittal Sinus of the Human Brain. Tomography, 2018. 4(3): p. 99-104.
8. Park, S.H. and T.Q. Duong, Brain MR perfusion-weighted imaging with alternate ascending/descending directional navigation. Magn Reson Med, 2011. 65(6): p. 1578-91.
9. Park, S.H., et al., Suppression of effects of gradient imperfections on imaging with alternate ascending/descending directional navigation. Magn Reson Med, 2012. 68(5): p. 1600-6.
Figures