0643
The driving force of glymphatics: influence of the cardiac cycle on CSF-mobility in perivascular spaces in humans1Leiden University Medical Center, Leiden, Netherlands, 2Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 3University Medical Centre Utrecht, Utrecht, Netherlands, 4Department of Biomedical Engineering & Physics, Amsterdam University Medical Center, Amsterdam, Netherlands
Synopsis
Recently, flow of cerebrospinal fluid (CSF) has been shown to play an important role in the waste clearance of the brain, ushering in the concepts of glymphatics and intramural periarterial drainage. Despite the importance of brain waste removal, the exact clearance mechanisms such as its driving force are still poorly understood and remain highly controversial. In this study, we image how the cardiac cycle influences the CSF-mobility in the human brain, in the perivascular spaces of the basal ganglia as well as in large CSF-filled spaces, i.e. ventricles and subarachnoid space around large arteries.
Introduction
Research into brain waste clearance and the concept of glymphatics has gained increasing attention and lead to different theories, with CSF-flow as a common denominator1,2. One of the theories proposes cardiac pulsations as main driving force of CSF-flow along the perivascular pathway3 . Whereas CSF-flow in the ventricles and aqueduct is well studied, it is unknown whether cardiac pulsations also affect CSF-mobility in perivascular spaces, where clearance is supposed to take place. Animal experiments only observed pulsatile CSF in the subarachnoid liquid around large arteries3,4, limited on the one hand by the superficial penetration depth of microscopes and by the low MRI-resolution on the other.Recently, we non-invasively measured CSF-mobility along perivascular spaces in the human brain5. Here, we study the effect of the cardiac cycle on CSF-mobility in perivascular spaces of the basal ganglia as well as in large CSF-filled spaces, i.e. ventricles and subarachnoid space around large arteries.
Methods
Five healthy subjects were scanned at 7T (Achieva, Philips, The Netherlands) using a 32-channel receive coil. All provided written informed consent for this IRB-approved study.MRI scans
We introduced a 17x accelerated pseudo-random undersampling scheme into our original T2-prepared turbo spin echo (TSE) sequence5 using the PROUD software patch6. This enabled to reach full brain coverage, while maintaining parameters that enable the isolation of the CSF signal (0.45mm3 isotropic, TE/TR=497/3486ms, acquisition time Tacq=5min30 per full-brain volume and gradient strength/direction). To study the influence of the cardiac cycle on the measured CSF signal, the heart rate was recorded using a peripheral pulse unit (PPU).
Dataset 1: To optimize our protocol, we acquired two non-crushed and one crushed scan with velocity-encoding gradients of 5mm/s included in the T2-preparation in two subjects. These gradients were applied in the right-left direction in order to specifically evaluate the effect of the cardiac cycle on the left-right oriented subarachnoid CSF around the middle cerebral artery (MCA) (Tacq,total=17min).
Dataset 2: In three subjects, one scan without and six scans with crushers (5mm/s) applied in different directions were acquired (Tacq,total=40min), allowing for fitting a full tensor model.
Reconstruction and post-processing
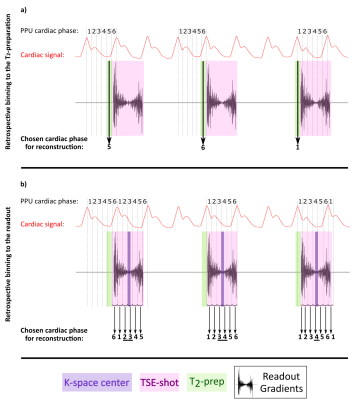
After acquisition, the k-space profiles were retrospectively binned in 6 cardiac phases. We evaluated two separate ways of binning the data for dataset 1:
- the cardiac phase during the T2-preparation preceding the TSE-shot was taken as reference and the signal in the subsequent TSE-shot was considered to be dependent on this specific cardiac phase (Figure 1a).
- the cardiac cycle of each separate k-space profile was measured, independently of the cardiac phase of the T2-preparation (Figure 1b).
Results
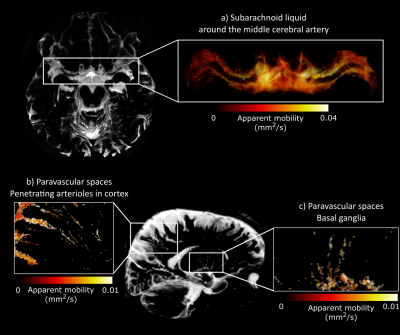
The implemented compressed-sense readout enabled measurements of CSF-mobility in different brain regions of the entire human brain within reasonable scan time (Fig.2).When binning the data in different cardiac phases, the normalized CSF-signal varies with the cardiac cycle both in the crushed and the non-crushed data (Fig.3). Both binning strategies exhibit the same dependency with the cardiac cycle, yet with a consistent phase shift.
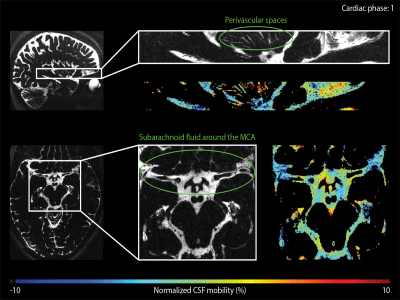
The CSF-mobility is clearly dependent on the cardiac cycle in large CSF-filled spaces such as in the ventricles or in the subarachnoid liquid around the MCA (Fig.4a). Such pulsations are also observed in perivascular spaces in the basal ganglia (Fig.4b), but the observed pattern is different: whereas the larger CSF-filled spaces exhibit only a single peak in the cardiac cycle, the perivascular spaces present two peaks.
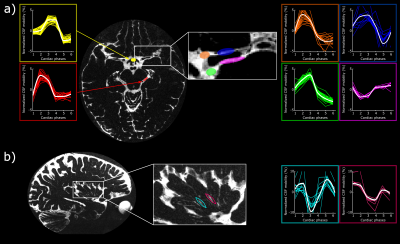
The time of the CSF-mobility peak(s) with respect to the cardiac cycle varies spatially (Fig.5, animated Gif).
Discussion
Since CSF-mobility is encoded by varying the crusher gradients in the T2-preparation, the effect of the cardiac cycle on the CSF-mobility was expected to be mainly dependent on the heart phase during the T2-preparation. However, the TSE-shot following each T2-preparation lasts 982ms, during which additional heart beats occur (Fig.1). These also affect the CSF-signal, as observed in the (non)-crushed data (Fig.3). The observed phase shift between the two reconstructions is equal to half the TSE-shot duration, which is consistent with the time of acquisition of k0 (Fig.1). By normalizing the crushed data with respect to the non-crushed (as done when calculating CSF-mobility), such readout effects will cancel out.Our study shows for the first time that a pulsatile effect is present in human perivascular spaces of small arteries. Without such cardiac cycle dependent mobility fluctuations, the cardiac cycle would be excluded as a potential driving force. The observed dual peaks could potentially indicate mixing mechanisms instead of net flow, but our current setup is not suitable to differentiate between these two processes.
Acknowledgements
This work is part of the research programme Innovational Research Incentives Scheme Vici with project number 016.160.351, which is financed by the Netherlands Organisation for Scientific Research (NWO). It was also partially financed by the Benelux-ISMRM chapter (Women in MR award).References
1. Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. (2012). doi:10.1126/scitranslmed.3003748
2. Albargothy, N. J. et al. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. (2018). doi:10.1007/s00401-018-1862-7
3. Mestre, H. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. (2018). doi:10.1038/s41467-018-07318-3
4. Harrison, I. F. et al. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife (2018). doi:10.7554/eLife.34028
5. Hirschler, L. et al. High resolution T2-prepared MRI enables non-invasive assessment of CSF flow in perivascular spaces of the human brain. in Proceedings of the 28th Annual Meeting of ISMRM, Montréal, Canada, 2019. Abstract 0746.
6. Mazzoli, V. et al. Accelerated 4D phase contrast MRI in skeletal muscle contraction. Magn. Reson. Med. (2018). doi:10.1002/mrm.27158
7. Uecker, M., Tamir, J. I., Ong, F. & Lustig, M. The BART Toolbox for Computational Magnetic Resonance Imaging. in ISMRM (2016). doi:10.5281
8. Klein, S., Staring, M., Murphy, K., Viergever, M. A. & Pluim, J. P. W. Elastix: a toolbox for intensity based medical image registration. IEEE Trans. Med. Imaging 29, 196–205 (2010).
Figures