0641
MR Molecular Imaging of EDB-Fibronectin for Precision Imaging of Oral Squamous Cell Carcinoma1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Oral squamous cell carcinoma (OSCC) has maintained poor prognosis due to its aggressive nature and lack of targetable biomarkers for early and accurate detection. We have developed a targeted MRI contrast agent specific to extradomain-B fibronectin (EDB-FN), an extracellular matrix protein closely associated with tumor aggressiveness. Immunohistochemical analysis revealed strong EDB-FN expression in OSCC with minimal expression in normal tongue tissue. MR molecular imaging with our targeted contrast agent demonstrated the ability for differential contrast enhancement of aggressive OSCC tumors, underscoring the potential for using EDB-FN as a targetable biomarker for precision molecular imaging of OSCC.
Introduction
Oral squamous cell carcinoma (OSCC) is the most common malignant neoplasm of the oral cavity, representing up to 95% of all malignancies of the head and neck1,2. Despite improvements in therapeutic options, the 5-year survival rate of patients with OSCC has not experienced significant improvement3. The overall survival rate is inversely related to tumor stage, up to 90% for Stage I tumors to as low as 10% for Stage IV tumors4. The poor prognosis associated with OSCC stems from aggressive local invasion and metastasis, resulting in high recurrence rates after surgical resection, and lack of targetable biomarkers for early detection1,3,4. It is therefore imperative to develop molecular imaging technology based on tumor-specific biomarkers of OSCC to facilitate non-invasive detection, precision imaging, and diagnosis of primary tumors and metastases to improve clinical outcomes.The tumor microenvironment plays a significant role in tumor initiation, progression, and metastasis5. Fibronectin, an abundant protein in the extracellular matrix, often experiences upregulation in tumors and has been associated with tumor invasion, metastasis, and therapy resistance6. An alternatively spliced isoform of fibronectin involved in neovascularization and cellular motility, extradomain-B fibronectin (EDB-FN), has been shown to be upregulated in many aggressive forms of cancer with little to no expression in normal adult tissues7. It is therefore a promising biomarker for molecular imaging of aggressive tumors. We have developed a novel targeted MRI contrast agent utilizing an EDB-FN-specific peptide probe, ZD2, to target EDB-FN in the tumor microenvironment for detection and precision imaging of patient tumors8-11. This research aims to determine the potential of magnetic resonance molecular imaging (MRMI) of EDB-FN for detection and differential diagnosis of aggressive OSCC.
Methods
To determine the prevalence of EDB-FN in OSCC, formalin-fixed paraffin-embedded human OSCC and normal tongue tissues were procured through the Human Tissue Procurement Facility at Case Western Reserve University. Samples were prepared using citrate buffer in a pressure cooker at 125oC for 30 seconds for antigen retrieval and stained with an EDB-FN-specific monoclonal antibody, G4, for immunohistochemistry. OSCC cell lines, CAL27 and SCC4, were obtained from the American Type Culture Collection. Cultures were grown atop Matrigel to facilitate ECM and 3D spheroid formation. The expression of EDB-FN was analyzed on the mRNA level using quantitative real-time PCR (qRT-PCR), and on the protein level using an EDB-FN-specific fluorescent-labeled peptide probe, ZD2-Cy5.5, with confocal fluorescence microscopy9. For MRMI, nude mice were subcutaneously inoculated on the right flank with 4x106 CAL27 or SCC4 cells and monitored for 4 weeks. T1-weighted MR images were obtained with a 2D spin-echo sequence pre- and post- injection of 0.04 mmol/kg ZD2-Gd(HP-DO3A) using a 3T MRSolutions small animal scanner. Prohance at the clinical dose of 0.1 mmol/kg was used as the control. Contrast-to-noise ratio (CNR) was calculated using muscle as the control.Results and Discussion
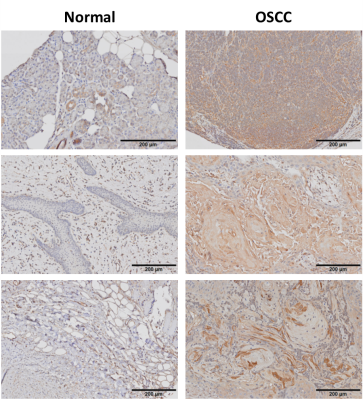
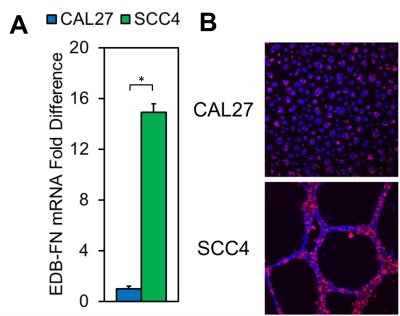
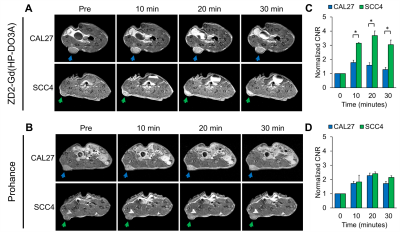
Immunohistochemical analysis of human OSCC samples displayed markedly increased staining intensity relative to normal tongue tissue (Fig 1), suggesting differential expression of EDB-FN in cancerous and non-cancerous tongue tissue for targeted imaging in OSCC. EDB-FN mRNA expression in CAL27 and SCC4 cell lines measured with qRT-PCR revealed significantly elevated EDB-FN expression in SCC4 (Fig 2a). When grown in 3D culture, SCC4 experiences significantly stronger ZD2-Cy5.5 staining than CAL27 (Fig 2b), corroborating the qRT-PCR results on the protein level. SCC4 formed large proliferative networks compared to the punctate spheroids of CAL27 (Fig 2b), suggesting that SCC4 presents a more aggressive phenotype than CAL27.MRMI of OSCC xenografts after ZD2-Gd(HP-DO3A) administration gave robust signal enhancement in the SCC4 tumors and less, but still significant enhancement in CAL27 tumors (Fig 3a), consistent with in vitro data for EDB-FN expression. There was little signal enhancement in CAL27 and SCC4 tumors when using Prohance (Fig 3b). Quantitative analysis of CNR enhancement revealed at least 3-fold increase in CNR for SCC4 with the targeted agent up to 30 minutes post-injection, which was significantly higher than CAL27 at all measured time points (Fig 3c). There was no significant difference in CNR enhancement with Prohance (Fig 3d). This data demonstrates that ZD2-Gd(HP-DO3A) can differentially enhance aggressive tumors at a much lower gadolinium dose than the current clinical agents.
Conclusion
EDB-FN is a promising diagnostic biomarker for OSCC. Our work demonstrates the potential for detection and precision imaging of aggressive OSCC tumors using MRMI and a peptide-targeted MR contrast agent specific to EDB-FN. Our work also demonstrates the ability to perform effective MRMI at a significantly reduced dose to reduce dose-dependent side effects of the targeted contrast agent.Acknowledgements
This research was supported by National Institutes of Health grants R01CA211762 and R44CA199826.References
[1] Taghavi N and Yazdi I. Prognostic factors of survival rate in oral squamous cell carcinoma: clinical, histologic, genetic and molecular concepts. Arch Iran Med. 2015;18(5):314-319.
[2] Wang B, Zhang S, Yue K, and Wang XD. The recurrence and survival of oral squamous cell carcinoma: a report of 275 cases. Chin J Cancer. 2013;32(11):614-618.
[3] Markopoulos AK. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126-130.
[4] Silva SD, Hier M, Mlynarek A, et al. Recurrent oral cancer: current and emerging therapeutic approaches. Front Pharmacol. 2012;3:149.
[5] Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761-773.
[6] Wang JP and Hielscher A. Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer. 2017;8(4):674-682.
[7] Han Z and Lu ZR. Targeting fibronectin for cancer imaging and therapy. J Mater Chem B. 2017;5(4):639-654.
[8] Han Z, Zhou Z, Shi X, et al. EDB fibronectin specific peptide for prostate cancer targeting. Bioconjug Chem. 2015;26(5):830-838.
[9] Han Z, Cheng H, Parvani JG, et al. Magnetic resonance molecular imaging of metastatic breast cancer by targeting extradomain-B fibronectin in the tumor microenvironment. Magn Reson Med. 2018;79(6):3135-3143.
[10] Han Z, Li Y, Roelle S, et al. Targeted contrast agent specific to an oncoprotein in the tumor microenvironment with the potential for detection and risk stratification of prostate cancer with MRI. Bioconjug Chem. 2017;28(4):1031-1040.
[11] Ayat NR, Qin JC, Cheng H, et al. Optimization of ZD2 peptide targeted Gd(HP-DO3A) for detection and risk-stratification of prostate cancer with MRI. ACS Med Chem Lett. 2018;9(7):730-735.
Figures