0634
Deuterium MR spectroscopy to probe Krebs cycle metabolism of the in-vivo heart1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
We investigated whether deuterium MR spectroscopy using 2H-labeled glucose can be used to assess Krebs cycle metabolism in the in-vivo heart. Localized 2H spectra were acquired from a slice containing the whole heart and from a voxel containing only the left ventricle of the heart using a temporal resolution of ~12 min following a bolus injection of [6,6′-2H2]glucose. Single voxel spectra show the production of labelled Glutamate/Glutamine (Glx) ˜36 min after the administration of the glucose. The Glx concentration reflects Krebs cycle activity which holds potential to probe various metabolic states of the heart.
Introduction
Deuterium (2H) MR has been proposed and applied to map metabolite concentrations in vivo without the use of hyperpolarization1. The technique relies on the administration of [6,6′-2H2]glucose and subsequent 2H MR acquisition. Since 2H signal intensities can be normalized to the endogenous semi-heavy water (HDO) concentration, quantification of 2H labelled metabolites is feasible. Here we present our initial experience with localized and dynamic 2H MR of the in-vivo heart.Methods
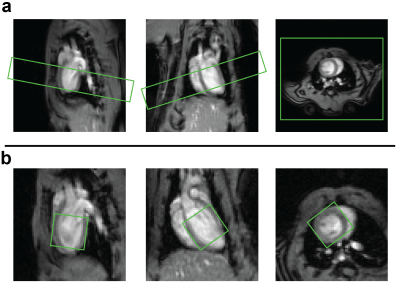
Two healthy female Wistar rats, approximately 4 months of age, weighting 296 g and 292 g were measured in a 9.4 T MR scanner (Bruker Biospin, Ettlingen, Germany) equipped with a 1H volume Tx/Rx coil (Bruker) and a 20 x 30 mm2 home-built 2H surface Tx/Rx coil placed on the chest of the rat. The animal experiments were performed according to the Swiss Federal Act on Animal Protection and were approved by the Cantonal Veterinary Office Zurich.Anatomical reference imaging of the heart was followed by B0 field mapping. The region of interest (slice or voxel) was subsequently shimmed using a first order optimization protocol based on the acquired B0 map. In the first animal a 10 mm slice containing the whole heart, tilted to minimize contamination from liver and lungs (Figure 2a), was prescribed for 2H MR. Slice-selective excitation was achieved with a sinc pulse in combination with an oblique gradient. In the second animal, single-voxel spectroscopy was performed using the ISIS method2 to produce spectra of a 10 mm isotropic voxel (Figure 1b) containing the left ventricle of the heart. In both cases one reference spectrum was acquired (number of points = 256, TR = 300 ms, bandwidth = 2000 Hz, flip angle = 90°, number of averages = 1600) before the injection of 2 g per kg bodyweight [6,6′-2H2]glucose (Sigma Aldrich, Switzerland) in saline solution into the tail vein. The spectra were acquired with cardiac gating but without respiratory gating. The gating prolonged the acquisition time per spectrum from 8 min to approximately 12 min. Seven spectra were acquired following the injection of the [6,6′-2H2]glucose. Individual peaks were fitted using prior knowledge of the chemical shift and the relative linewidths using an implementation of the AMARES algorithm3 in MATLAB (MathWorks, Natick, USA). Concentrations were calculated by referencing the metabolite amplitudes to the amplitude of the pre-injection natural abundance HDO which was assumed to be 10.12 mM 1 and by correcting for the numbers of 2H nuclei per molecule (one deuteron in HDO, two deuterons in Glx, in lactate and in glucose). Thereby absolute quantification of the deuterated metabolite concentration was achieved.
Results
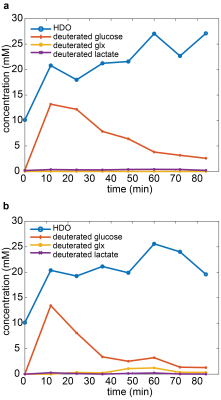
Both, the slice and ISIS localized MR measurement allowed to detect the time evolution of the metabolite concentrations (Figure 2). The glucose and the HDO peak could be well separated (Figure 3). The signal-to-noise ratio (SNR) of the slice selective spectra was higher than that of the single voxel spectra. The linewidth of the peaks in the slice-selective spectra was broader compared to the ISIS experiment (HDO peak linewidth: 99 vs 37 Hz, glucose peak linewidth: 77 vs 15 Hz). In the single-voxel spectra (Figure 2b and 3b) Glutamate / Glutamine (Glx) could be detected. The Glx concentration from minute 36 to 60 after the injection was approximately 1.2 mM.Discussion
The present study has demonstrated quantification of Glutamate / Glutamine using 2H MR in the in-vivo heart for the first time, which has previously been reported in brain1,4. In comparison to hyperpolarized MR using [2-13C]pyruvate5, which allows probing [5-13C]glutamate, the 2H based approach has the advantage that metabolite concentrations can be quantified using the internal HDO reference. Because these metabolites are in exchange with the Krebs cycle intermediate alpha Ketoglutarate they can be used to assess Krebs cycle activity. While the present work demonstrates basic feasibility, further development may open up a range of applications to probe Krebs cycle metabolism in diabetes, ischemia, hypertrophy and heart failure6–8.Acknowledgements
No acknowledgement found.References
1. De Feyter HM, Behar KL, Corbin ZA, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018;4(8):eaat7314.
2. Ordidge RJ, Connelly A, Lohman JAB. Image-Selected in Vivo Spectoscropy (ISIS). A New Technique for Spatially Selective NMR Spectroscopy. J. Magn. Reson. 1986;66:283–294.
3. Vanhamme L, Van Den Boogaart A, Van Huffel S. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge. J. Magn. Reson. 1997;129(1):35–43.
4. Lu M, Zhu X-H, Zhang Y, Mateescu G, Chen W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 2017;37(11):3518–3530.
5. Josan S, Park JM, Hurd R, et al. In vivo investigation of cardiac metabolism in the rat using MRS of hyperpolarized [1-13C] and [2-13C]pyruvate. NMR Biomed. 2013;26(12):1680–1687.
6. Malloy CR, Merritt ME, Dean Sherry A. Could 13C MRI assist clinical decision-making for patients with heart disease? NMR Biomed. 2011;24(8):973–979.
7. Schroeder MA, Clarke K, Neubauer S, Tyler DJ. Hyperpolarized magnetic resonance: A novel technique for the in vivo assessment of cardiovascular disease. Circulation 2011;124(14):1580–1594.
8. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005;85(3):1093–1129.
Figures