0632
Hyperpolarized 13C Pyruvate Imaging of Glioblastoma Patients1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Neurosurgery, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3GE Healthcare, GE Healthcare, Dallas, TX, United States, 4Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Electrical Engineering, University of Texas Dallas, Richardson, TX, United States
Synopsis
Noninvasive tumor characterization is extremely beneficial for brain tumor patients for establishing surgical procedure and treatment plans. In this study, we imaged newly diagnosed glioblastoma patients using hyperpolarized [1-13C]pyruvate few days prior to surgical procedures and compared the imaging and biopsy results to evaluate the diagnostic values of hyperpolarized pyruvate imaging. Brain regions with increased 13C-lactate production are confirmed as glioblastoma from stereotactic tissue-biopsy. This pilot study with treatment-naïve or newly diagnosed brain tumor patients suggest that preoperative metabolic imaging with hyperpolarized [1-13C]pyruvate may have strong diagnostic value with potential to be an alternative method for tissue biopsy.
Introduction
Identifying tumor characteristics prior to surgical procedure and treatment planning is critical for brain tumor patients to avoid unnecessary craniotomy and to establish effective target regions for surgical resection and radiation. Although MRI and PET provide various tissue characteristics such as vascularization, cellular density and glucose uptake, stereotactic brain biopsy is still critical for classifying the tumor type and determining the brain regions for tumor resection. Carbon-13 (13C) MR imaging with an intravenous injection of hyperpolarized 13C-labeled substrates provides metabolic information of how the substrates can be utilized in cellular level in vivo. In particular, hyperpolarized [1-13C]pyruvate is useful to assess altered metabolic fates of pyruvate in cancer: upregulated lactate dehydrogenase (LDH) activity [1] and decreased pyruvate dehydrogenase (PDH) flux [2]. Since the first clinical translation in patients with prostate cancer [3], imaging with hyperpolarized [1-13C]pyruvate has been utilized in several applications in human including patients with brain tumors [4]-[6]. The previous brain metabolism studies were with either patients who underwent chemotherapy and/or radiation therapy or patients with metastatic brain tumors. In this study, we imaged untreated glioblastoma patients using hyperpolarized [1-13C]pyruvate few days prior to surgical procedures (biopsy, resection) and compared the imaging and biopsy results to evaluate the diagnostic role of hyperpolarized pyruvate imaging. As far as we know, this is the first study of imaging treatment-naïve glioblastoma patients (patient#1 only) using hyperpolarized 13C-pyruvate.Methods
We recruited two newly diagnosed and untreated brain tumor patients with potential glioblastoma, based on 1H MRI. Each patient was scanned 2-3 days prior to tissue biopsy/tumor resection using a 60-min 13C-1H mixed MR imaging protocol. A 13C/1H dual-frequency head coil was used throughout the MR session [7]. The imaging protocol includes two injections of hyperpolarized [1-13C]pyruvate with a time interval of at least 30 min between the injections. The polarization procedure and the dosage of pyruvic acid are consistent with prior human studies [3], and the protocol was described in our previous healthy subject studies with an addition of contrast-enhanced (CE) T1-weighted 1H imaging at the end of the session [8]. Briefly, two-dimensional T2-weighted FLAIR images are acquired to localize the tumor mass, followed by a two-dimensional 13C spiral chemical shift imaging (CSI; FOV = 24 × 24 cm2, matrix size = 16 × 16, slice thickness = 2.5-3.0 cm, 7 spatial interleaves, spectral width = 817 Hz, variable flip angle up to 30o for each time point, 16 dynamic scans, temporal resolution = 5 sec). The 13C acquisition started 3 sec after the start of pyruvate injection. After the first 13C imaging, a series of 1H MRI (ASL, SWI, T2-weighted FSE, and pre-Gd T1-weighted SE) are acquired for 30 min. Finally, the dynamic 13C spiral CSI was repeated with another injection of hyperpolarized [1-13C]pyruvate, followed by Gd-enhanced T1-weighted SEResults and Discussion
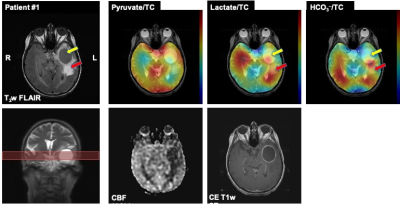
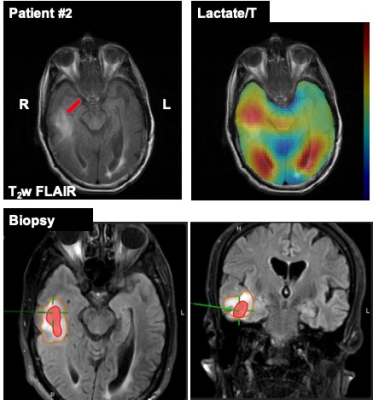
The first patient (66 years old, female) had a large cyst in the left temporal lobe with enhancing tumor margin in 1H MRI (Fig.1). The cyst in the center of the enhancing ring contained watery fluid and did not show any 13C signals as the perfusion was limited (as confirmed by 1H ASL and CE T1-weighted SE). Two specimens were collected through biopsy: enhancing tumor margin and anterior lateral tumor margin in the left temporal lobe. Immunohistochemistry confirmed that the enhancing tumor margin was high-grade glioblastoma (WHO grade IV) with wild-type isocitrate dehydrogenase (IDH) and that the anterior lateral tumor margin did not contain tumor histologically. In hyperpolarized 13C images, all the metabolite signals were low in the cyst and the anterior lateral tumor margin. The posterior tumor margin and the left occipital region, however, showed enhancing 13C lactate signals as compared to the contralateral hemisphere, indicating possible tumor progression towards the corpus callosum. No tissue sample was collected from the left posterior tumor margin for confirmation. The second patient (69 years old, male) had glioblastoma in the left parietal region previously. The tumor was removed 2.5 years ago, and treated with chemo/radiation therapy. While there is no recurrence at the site, a new asymptomatic tumor in the right temporal lobe (Fig.2) was identified recently and has progressed despite chemotherapy (no radiation). The biopsy confirmed that the anterior tumor in the right temporal lobe is mitotically active glioblastoma, which is a common characteristic of recurrent tumors, while tissue samples from lateral temporal FLAIR and lateral temporal white matter did not contain glioblastoma, indicating heterogeneity of the tumor. The hyperpolarized 13C imaging showed increased lactate labeling in the anterior side of the tumor. From the both patients, the biopsy-proven glioblastoma tissues were consistent with the regions that showed increased lactate production from hyperpolarized 13C-pyruvate. Improving spatial resolution will provide more detailed and precise noninvasive assessment of the tumor metabolism.Conclusion
Brain regions with increased 13C-lactate production from hyperpolarized 13C-pyruvate are confirmed as glioblastoma from stereotactic tissue-biopsy. The pilot study with treatment-naïve brain tumor patients suggest that preoperative metabolic imaging with hyperpolarized [1-13C]pyruvate may have strong diagnostic value with potential to be an alternative method for tissue biopsy.Acknowledgements
Annette G. Strauss Center for Neuro-Oncology; The Texas Institute of Brain Injury and Repair; The Mobility Foundation; National Institutes of Health of the United States (P41 EB015908, S10 OD018468); The Welch Foundation (I-2009-20190330); UT Dallas Collaborative Biomedical Research Award.References
[1] I. Park, P. E. Z. Larson, M. L. Zierhut, S. Hu, R. Bok, T. Ozawa, J. Kurhanewicz, D. B. Vigneron, S. R. Vandenberg, C. D. James, and S. J. Nelson, “Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors.,” Neuro-oncology, vol. 12, no. 2, pp. 133–144, Feb. 2010.
[2] J. M. Park, L. D. Recht, S. Josan, M. Merchant, T. Jang, Y.-F. Yen, R. E. Hurd, D. M. Spielman, and D. Mayer, “Metabolic response of glioma to dichloroacetate measured in vivo by hyperpolarized (13)C magnetic resonance spectroscopic imaging.,” Neuro-oncology, vol. 15, no. 4, pp. 433–441, Apr. 2013.
[3] S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, P. E. Z. Larson, A. L. Harzstark, M. Ferrone, M. Van Criekinge, J. W. Chang, R. Bok, I. Park, G. Reed, L. Carvajal, E. J. Small, P. Munster, V. K. Weinberg, J. H. Ardenkjaer-Larsen, A. P. Chen, R. E. Hurd, L.-I. Odegardstuen, F. J. Robb, J. Tropp, and J. A. Murray, “Metabolic imaging of patients with prostate cancer using hyperpolarized [1-¹³C]pyruvate.,” Sci Transl Med, vol. 5, no. 198, p. 198ra108, Aug. 2013.
[4] I. Park, P. E. Z. Larson, J. W. Gordon, L. Carvajal, H.-Y. Chen, R. Bok, M. Van Criekinge, M. Ferrone, J. B. Slater, D. Xu, J. Kurhanewicz, D. B. Vigneron, S. Chang, and S. J. Nelson, “Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies.,” Magn Reson Med, Jan. 2018.
[5] V. Z. Miloushev, K. L. Granlund, R. Boltyanskiy, S. K. Lyashchenko, L. M. DeAngelis, I. K. Mellinghoff, C. W. Brennan, V. Tabar, T. J. Yang, A. I. Holodny, R. E. Sosa, Y. W. Guo, A. P. Chen, J. Tropp, F. Robb, and K. R. Keshari, “Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor Patients.,” Cancer Res., p. canres.0221.2018, May 2018.
[6] B. Geraghty, C. Lee, A. Chen, N. Bragagnolo, W. Perks, H. Soliman, and C. Cunningham, “Partial Fourier Reconstruction of Hyperpolarized 13C EPI in the Human Brain.,” ISMRM. #4280, 2019.
[7] J. Ma, R. Hashoian, C. Sun, S. Wright, A. Ivanishev, R. Lenkinski, C. Malloy, A. Chen, and J. M. Park, “Development of 1H/13C RF head coil for hyperpolarized 13C imaging of human brain.,” ISMRM. #568, 2019.
[8] J. M. Park, J. Liticker, C. Harrison, G. Reed, T. Hever, J. Ma, R. Martin, D. Mayer, R. Hashoian, C. Madden, M. Pinho, and C. Malloy, “Feasibility and reproducibility of imaging brain metabolism using hyperpolarized 13C pyruvate in humans.,” ISMRM. #4311, 2019.
Figures