0626
Image-guided tumor resection of head and neck carcinoma (HNSCC) in rabbit models with targeting MRI-Raman nanoprobe1Shanghai Ninth People’s Hospital, Shanghai, China, 2United Imaging Healthcare, Shanghai, China, 3Fudan University, Shanghai, China
Synopsis
Accurately defining infiltrative tumor margin is extremely crucial for complete resection and avoiding mistaken removal of normal tissue. Currently, pre-operative MRI imaging is the most widely-used strategy for defining tumor margin. However, there are plenty of insurmountable disadvantages in terms of accuracy, low resolution, mismatch and so on. This research aims to develop one targeting MRI-Raman nanoprobe able to pre-operatively and intra-operatively evaluate infiltrative margin of head and neck carcinoma (HNSCC) and real-timely guide tumor resection. The results showed that the nanoprobe greatly benefits the complete resection of HNSCC. As a result, the prognosis of tumor-bearing rabbits were considerably improved.
Introduction
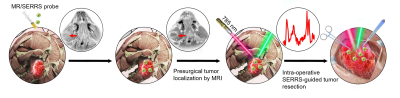
As the infiltrative character of many tumors can notoriously lead to a lot of clinical outcomes including the recurrence, poor prognosis and so on. Accurate defining the infiltrative margin that is extremely crucial for thorough surgical resection as well as preventing the mistaken removal of normal tissue has gained the attention of innumerable researchers.1-4 During the clinical practice, the pre-operative MRI imaging, with a lot of insurmountable weaknesses containing low resolution, low sensitivity, mismatch, unsatisfied accuracy and so on, is the most widely-used strategy for defining tumor margin. Having the priorities being complementary to MRI such as ultra-high sensitivity , multiplexing capability sourced from fingerprint-like spectrum and more, Raman Imaging has recently displayed fascinating clinical potential in intra-operatively guiding tumor resection.5 However, as far as we are aware, Raman imaging was hardly applied in combination with MRI for defining the infiltrative tumor margin of HNSCC. This research aims to develop one multiple model–MR-Raman targeting nanoprobe to improve surgical resection of HNSCCs (Figure 1).Methods
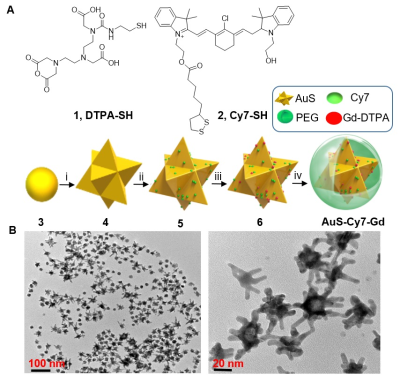
Synthesis and Characterization: The gold nanostar (AuS) based multiple model nanoprobe termed as AuS-Cy7-Gd was synthesized according to our previous work.1 DTPA-Gd was fabricated on the surface of gold nanostar for serving as MRI reporters. Cy7-SH is a type of heptamethine cyanine molecule, which was applied for serving as Raman reporter. Establishment of VX2 Tumour bearing rabbit Modal: VX2 tumour models were established according to our previous study.1 Pre-operatively imaging infiltrative tumor margin of HNSCC: MR measurements was performed with the 3-T MR scanner (MAGNETOM Verio, Siemens AG, Germany). The main protocol includes both pre- and post-contrast coronal T1-weighted imaging (T1WI). The detailed parameters are as followings: FOV: 120×120 mm2, matrix: 256×160, TR/TE: 350/15 ms, slice thickness: 1.0 mm, NEX: 2. The contrast-enhanced T1WI image was obtained 20 min post-intravenous administration of AuS-Cy7-Gd via the ear vein. The T1 relaxation rate of nanoprobe was measured with 1.5 T MR scanner (uMR560, Shanghai United Imaging Healthcare, China). Intra-operatively guiding HNSCC resection with the aid of targeting nanoprobe: All of the HNSCC bearing rabbits were randomly divided into two groups (n = 10 for each group) as follows: (1) the nanoprobe-guided tumour surgery group, referring to the group where intraoperative Raman guided surgical resection of primary tumour; and (2) the white-light-illumination surgery group, referring to the group for which tumour resection was performed only under white-light illumination but without Raman imaging guidance;Results
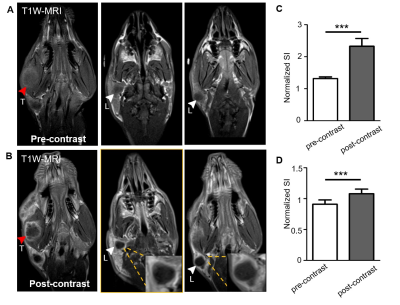
The multifunctional MRI-Raman nanoprobe developed in this work, termed as AuS-Cy7-Gd, had an average diameter of 60 nm. The synthesis of the nanoprobe is described in Figure 2. Preoperative MR Delineation of infiltrative margins:Prior to administration AuS-Cy7-Gd, the tumour margins were blurred and could not be well defined on the T1WI MR images (Figure 3). In addition to the primary tumour, an enlarged homolateral lymph node was visualized on the right side of the neck, with heterogeneous hypo-intensities. Compared to the pre-contrast images, an obvious enhancement (80% increase of normalized SI) of the tumour marginal areas delineated the primary tumour more clearly on contrast-enhanced T1WI images, and infiltrative margins of the tumour were observed with contrast enhancement (Figure 3).
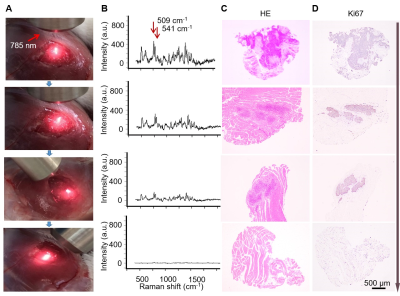
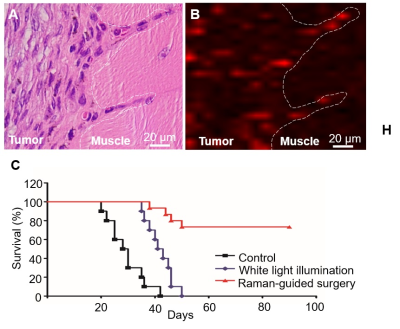
Intraoperative Raman delineation of infiltrative margins: Figure 4 illustrates the step-by-step Raman-guided surgical resection procedures. Corresponding Raman spectrum (panel B) of the illuminated area was synergized in the Raman-guided surgical resection process (panel A). Characteristic double Raman peaks at 509 cm-1 and 541 cm-1 were observed in tumour areas, representing the AuS-Cy7-Gd’s accurate targeting in the infiltrative tumor margin. Figure 5 illustrated that AuS-Cy7-Gd was able to accurately visualize the infiltrative tumor margin and improve the surgical prognosis of HNCSS bearing rabbit model.
Discussion
Based on Enhanced permeability and retention (EPR) effect6, with diameter of around 60 nm, the MR-Raman nanoprobe – AuS-Cy7-Gd can accurately target into tumor tissue especially in infiltrative tumor margin with strong angiogenesis effect, which endowed the AuS-Cy7-Gd with the ability of depicting the infiltrative tumor margin of HNSCCs. Moreover, with the MR reporters (Gd3+) and Raman reporters (Cy7-SH) fabricated on AuS surface, the AuS-Cy7-Gd can both help preoperatively imaging infiltrative tumor margin and intraoperatively guiding tumor resection with fingerprint-like spectrum of Cy7-SH (509 cm-1 and 543 cm-1). In the preoperative evaluation, the obvious enhancement in T1WI images provided the preliminary characterization of infiltrative tumor margin. Furthermore, accurately targeting into tumor tissue especially in tumor margin, the characteristic Raman signal of 509 cm-1 and 543 cm-1 of nanoprobe suggested the malignant lesions and infiltrative margins. Most importantly, according to the preoperative and intraoperative guidance sourced from MR-Raman multiple model nanoprobe–AuS-Cy7-Gd, much more clear delineation of tumor margin was achieved, which greatly improved the prognosis of tumor-bearing rabbit model compared to prognosis of tumor bearing rabbit without the guidance of nanoprobe.Conclusion
Overall, the bimodal MR-Raman probe with high targeting capability developed in this study enabled intraoperative visualization and image-guided resection of HNSCCs. The high sensitivity and tumour specificity between preoperative and intraoperative images of MRI and Raman imaging provide a new opportunity to improve the surgical prognosis of tumours with infiltrative behaviour.Acknowledgements
No AcknowledgementsReferences
1. Han L, Duan W, Li X, et al. Surface-Enhanced Resonance Raman Scattering-Guided Brain Tumor Surgery Showing Prognostic Benefit in Rat Models. ACS Appl Mater Inter 2019;11(17):15241-15250.
2. Crombe A, Le Loarer F, Stoeckle E, et al. MRI assessment of surrounding tissues in soft-tissue sarcoma during neoadjuvant chemotherapy can help predicting response and prognosis. Eur J Radiol 2018;109:178-187.
3. Iwata S, Araki A, Funatsu H, et al. Optimal surgical margin for infiltrative soft tissue sarcomas: Assessing the efficacy of excising beyond the infiltration. J Surg Oncol 2018;118(3):525-531.
4. Peeken JC, Molina-Romero M, Diehl C, et al. Deep learning derived tumor infiltration maps for personalized target definition in Glioblastoma radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2019;138:166-172.
5. Karabeber H, Huang R, Iacono P, et al. Guiding brain tumor resection using surface-enhanced Raman scattering nanoparticles and a hand-held Raman scanner. ACS Nano 2014;8(10):9755-9766.
6. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev 2013;65(1):71-79.
Figures