0625
Multivalent Gadolinium-decorated Peptides for Versatile Bioconjugation of Molecular MRI Probes
Nikorn Pothayee1, Deepak Sail2, Stephen Dodd1, Rolf Swenson2, and Alan Koretsky1
1Laboratory of Functional and Molecular Imaging, National Institutes of Health, Bethesda, MD, United States, 2Imaging Probe Development Center, National Institutes of Health, Rockville, MD, United States
1Laboratory of Functional and Molecular Imaging, National Institutes of Health, Bethesda, MD, United States, 2Imaging Probe Development Center, National Institutes of Health, Rockville, MD, United States
Synopsis
One of the most important goals of brain imaging is to define the anatomical connections within the brain. In addition to revealing normal circuitry, studies of neural connections and their transports can show rewiring and outgrowth during degeneration following brain injury and diseases. Ultrasensitive agents that can reveal neuroconnectivity and axonal transport dynamics in vivo will be very useful and allow for the interrogation of changes in brain connections and circuitry. In this work, we report two novel MR-visible neural tracers that can be used to visualize neuroconnectivity in vivo.
Introduction
There has been tremendous interest in developing MRI probes for detecting diseases, biological functions, and therapeutic responses. Common strategies to enhance relaxivities relies on (1) use of macromolecules and nanoparticles to carry a high payload of contrast agents, and (2) increase the longitudinal relaxivities (r1) per metal ion. However, in some circumstances that require tissue extravasation, molecular probes with large sizes may not be desirable due to limited penetration through the extracellular space. Importantly, attaching large macromolecules and nanoparticles to biomolecules may hamper their transport properties. For example, our group has reported a MRI-visible neurotracer based on GdDOTA-conjugated Cholera-toxin Subunit-B (CTB) and validated its applicability to visualize neuroconnections in vivo [1]. Effort to increase Gd per CTB with Gd dendrimers, however, resulted in the abolishment of neuronal transport. Increasing Gd moieties, while preserving properties of native compound by using a single conjugation site should improve upon detectability of this MR-visible neural tracer compound and expand its use for in vivo experiment. In this study, we propose using small peptides as a versatile scaffold for conjugation of multiple Gd to biomacromolecules. We demonstrate the applicability to conjugate this novel scaffold to CTB (retrograde neural tracer) and Dextran amine or DA (anterograde neural tracer) for use as sensitive in vivo MR-visible neural tracers.Methods
Synthesis: A peptide backbone consisting of alternating alanine and lysine(DOTA) amino acids were synthesized by utilizing standard Solid Phase Peptide Synthesis (SPPS). Peptides with 3 or 5 GdDOTAs were prepared (Figure 1). These were then complexed with Gd using gadolinium chloride. GdDOTA- peptide was reacted with excess disuccinimidyl suberate to yield NHS ester of the GdDOTA-peptide. NHS ester peptide was then conjugated with CTB and DA to yield the (GdDOTA)5-CTB and (GdDOTA)5-DA conjugates, respectively. Relaxivity Measurement: T1 and T2 relaxation times of the GdDOTA-peptide conjugates were measured on an 11.7T/31-cm horizontal bore magnet and a Bruker 4.7T/40-cm magnet. The specific relaxivities (r1) of the Gd-peptides were measured as follows. Samples were prepared at five different concentrations, and T1 values were measured for each concentration, which were then used for r1 calculations, respectively. Relaxivities were determined from the slope of concentration-dependent T1 changes. The Gd concentrations were based on the molar concentration of Gd atom measured by ICP. In Vivo Imaging: Three male Sprague-Dawley rats received brain injections of (GdDOTA)5-CTB. Two male Sprague-Dawley rats received (GdDOTA)5-DA. For the GdDOTA-peptide-CTB construct, injections were made to the somatosensory cortex in the forepaw area (S1FL) (AP 0.0 mm, ML 3.8 mm, and DV 2.9 mm). For GdDOTA-peptide-DA construct, injections were made to the ventral posteromedial nucleus (VPM) (AP 3.00 mm, ML 3.2 mm, and DV 6.0 mm), according to the Paxinos and Watson atlas [2]. A series of images were acquired to measure the transportation of the agents either from the S1FL to thalamus or thalamus to S1BF. Brain images were acquired on an 11.7T/31 cm horizontal bore magnet. A Magnetization Prepared Rapid Gradient Echo (MP-RAGE) sequence was used. 20 coronal slices with FOV= 2.56 × 2.56 cm, matrix 256 × 256, thickness = 0.5 mm (TR = 4000 ms, Echo TR/TE = 15/4 ms, TI = 1000 ms, number of segments = 4, and averages = 6) were used to cover the area of interest at 100 μm in-plane resolution.RESULTS AND DISCUSSION
Two multivalent Gd-conjugated oligopeptide scaffolds were synthesized. Both 3Gd-peptide and 5Gd-peptide exhibit excellent r1 relaxivities (Figure 2). 3Gd-peptide has relaxivities of 7.8 and 5.1 mM-1 s-1 per Gd at 4.7 and 11.7 T, respectively. The relaxivities of 5Gd-peptide are 8.2 and 6.0 mM-1 s-1 per Gd at 4.7 and 11.7 T, respectively. The r2/r1 ratio of the two compounds were relatively small in the range of 1.1-1.2 and 1.7-1.8 at 4.7 T and 11.7 T, respectively, suggesting that they are good T1-contrast agent for high-field MRI [3]. The 5Gd-peptide scaffolds were subsequently attached onto CTB and dextran amine to yield multivalent (GdDOTA)5-CTB and (GdDOTA)5-DA bearing multiple Gds tracer molecule (Figure 3). The relaxivities of the conjugates increase by approximately 2- to 3-fold relatively to the native GdDOTA-peptide. In vivo imaging with MRI show that both agents could be traced to the targeted areas of the brain. Injection of (GdDOTA)5-CTB into S1FL area led to retrograde transport to the ventral posterolateral nucleus (VPL) in the thalamus (Figure 4). Conversely, injection of (GdDOTA)5-DA into VPM area of thalamus led to anterograde transport to the barrel representation in the sensory cortex (S1BF) (Figure 5). The results show that the multivalent (GdDOTA)5-CTB and (GdDOTA)5-DA developed herein are an excellent retrograde and anterograde neurotracers in vivo, respectively.Conclusion
We have successfully synthesized multivalent gadolinium-decorated peptide scaffolds that possess high r1 relaxivities. GdDOTA-oligopeptide scaffold could also pave the way for attaching multiple MR-visible probes onto other bioactive molecules. In this work, GdDOTA-oligopeptide scaffolds were successfully conjugated onto classical neural tracers, CTB and DA. Potential applications of the multivalent GdDOTA-CTB will furnish a much richer in vivo diagram of brain circuitry. This in vivo approach could reveal changes occurring during plasticity induced by normal or abnormal physiology (e.g., axonal pruning or sprouting), in both central and peripheral nervous systems.Acknowledgements
This research was supported by the NINDS and NHLBI Intramural Research Programs of NIH.References
[1] Wu C. W., Vasalatiy O., Liu N., Wu H., Cheal S., Chen D.Y., Koretsky A. P., Griffiths G. L., Tootell R. B., Ungerleider L. G., (2011) Development of a MR-visible compound for tracing neuroanatomical connections in vivo. Neuron, 70, 229-243. [2] Paxinos, G.,Watson, C., (2007) The Rat Brain in Stereotaxic Coordinates, 6th Edition (Amsterdam: Elsevier). [3] Hagberg G. E, Scheffler K. (2013) Effect of r1and r2relaxivity of gadolinium-based contrast agents on the T1-weighted MR signal at increasing magnetic field strengths. Contrast Media Mol. Imaging, 8, 456-465.Figures
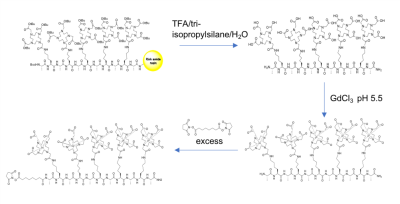
Synthesis
of multivalent GdDOTA-conjugated peptide scaffold
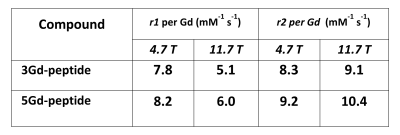
MR
relaxivities of 3Gd-peptide and 5Gd-peptide at 4.7 and 11.7 T
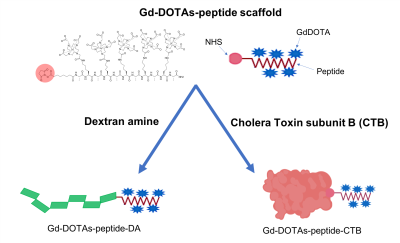
Bioconjugation
of NHS-activated GdDOTAs-peptide scaffold onto dextran amine and cholera toxin
subunit B through the amine groups on biomacromolecules.
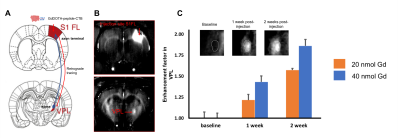
In
vivo demonstration
of retrograde tracing of 5GdDOTA-peptide-CTB. (A) Schematic representation of thalamocortical
connectivity between S1 forepaw (S1F1)-and VPL. (B) MR imaging shows signal
enhancement in VPL area following 1 week of injection of 5GdDOTA-peptide-CTB
into S1FL. (C) CTB shows dose- and time-dependent enhancement ratio in VPL (N =
3).
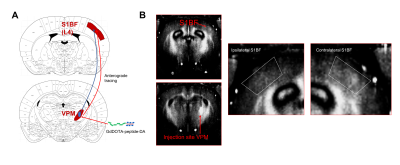
In
vivo demonstration
of anterograde tracing of GdDOTA-peptide-DA. (A) Schematic representation of
thalamocortical connectivity between S1 barrel field (S1BF) and VPM. (B) MR
imaging shows signal enhancement in S1BF area following 1 week of injection of
5GdDOTA-peptide-DA into VPM (N = 2).