0624
An MRI Method for Labeling and Imaging Decellularized Extracellular Matrix Scaffolds for Tissue Engineering1Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Translational Biology & Engineering Program, Ted Rogers Centre for Heart Research, Toronto, ON, Canada, 3Latner Thoracic Surgery Laboratories, Toronto General Hospital Research Institute, University Health Network, Toronto, ON, Canada, 4Biomedical Engineering Laboratory, University of Sao Paulo, Sao Paulo, Brazil, 5Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON, Canada, 6Latner Thoracic Surgery Laboratories, University of Toronto, Toronto, ON, Canada, 7Ontario Institute for Regenerative Medicine, Toronto, ON, Canada, 8Heart & Stroke/Richard Lewar Centre of Excellence for Cardiovascular Research, Toronto, ON, Canada, 9Edward S. Rogers Sr. Department of Electrical & Computer Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Extracellular matrix (ECM) forms the underlying complex structure of bodily tissues, for this reason, ECM has been greatly explored as an ideal scaffold for tissue engineering. To better understand and optimize scaffold-based therapies we require sensitive and non-invasive imaging techniques. In this study, a novel and facile method for labelling and imaging decellularized ECM scaffolds is presented. A series of tissue-specific (bladder, lung, and tracheal smooth muscle and cartilage) dECM scaffolds are labelled with a small molecule manganese porphyrin, MnPNH2. The labelled scaffolds are biocompatible and exhibit significant and sustained signal in vitro and in vivo.
Introduction
The ultimate goal in tissue engineering and regenerative medicine is building complex, three-dimensional (3D) tissue structures, even entire organs, for transplantation to obviate the need for donor transplant. To support these native structures tissue-specific dECM provides a viable scaffolding. It is a material which no synthetic alternative can identically replicate and is a perfect surrogate for replacement of native tissue in disease and injury. However, due to these similarities with native tissue, it poses a challenge for in vivo assessment with traditional non-invasive techniques. To enhance contrast and provide unambiguous identification in vivo a labelling method is required. This study is, to our knowledge, the first report of a method for labelling and imaging dECM scaffolds on MRI. We employ a positive-contrast manganese porphyrin (MnPNH2) agent for facile and efficient labelling of a range of decellularized tissue types.Methods
MnPNH2 was synthesized as previously reported1 and characterized by HPLC, flame AAS and mass spectroscopy to confirm purity of >95%. dECM scaffolds (pig bladder and trachea and murine lungs) were prepared according to a published protocol.2 dECM gels were prepared from pig bladders according to a published protocol utilizing enzymatic digestion with pepsin in acid solution.3 Gels were solidified by neutralization and warming to 37°C. The labeling protocol was optimized on bladder dECM, and imaging on a 3-Tesla clinical scanner was performed to assess reductions in T1 and T2 relaxation times. Quantitative T1-mapping was performed by inversion recovery at various inversion times, while T2 mapping was performed with a MS TSE with TR=2000 and 32 echos.1 In-vivo MRI was performed on labelled dECM gels injected in rat dorsum (adult female Sprague Dawley) to verify sensitivity of detection. After optimization, scaffolds and whole organs were labelled by incubation with 0.2mM MnPNH2 in PBS 1x (pH7.4) at 37°C overnight with shaking. Ex-vivo imaging was performed on all labelled grafts and organs using a 3-Tesla scanner. Toxicity assays for cell viability, metabolism, and proliferation were performed on human umbilical vein endothelial cells. The incorporation of MnPNH2 and its long-term retention in dECM were assessed with transmission electron microscopy and ultraviolet absorbance of eluted MnPNH2 over time.Results
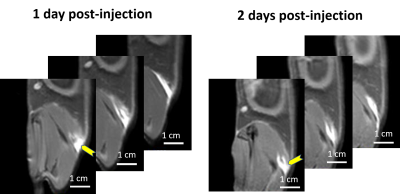
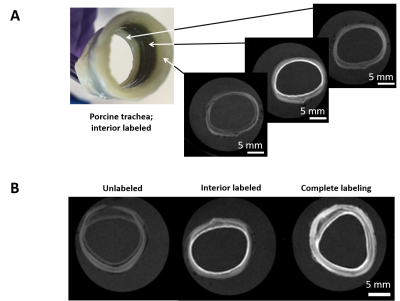
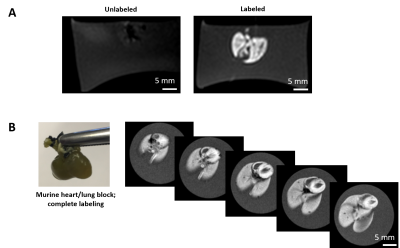
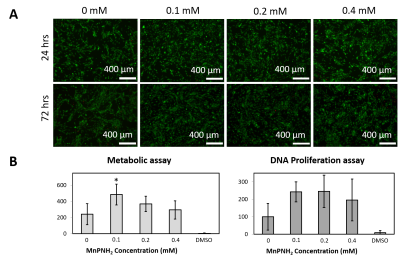
The dECM labeling protocol for the four different tissue types investigated in this study is shown in Figure 1. Initial labelling optimization was conducted with porcine bladder dECM scaffolds with a range of MnPNH2 concentrations from 0-4mM. A significant reduction from control (unlabeled scaffolds) in both T1 and T2 was observed with higher doses of MnPNH2 labeling (P < 0.05). Sensitivity of in-vivo scaffold detection on MRI is shown in Figure 2. dECM bladder gels labeled with 0.2mM of MnPNH2 injected in rat dorsum exhibit large contrast enhancement despite such a low dose of MnPNH2. Three consecutive imaging slices are included to highlight the intrinsic capability of MRI to track the shape of the dECM at different slice locations both one day and two days post-injection. Figures 3 and 4 illustrate decellularized and labeled whole tissue and organs. Photographs and MR images of the labeled decellularized porcine trachea (Fig.4) and labeled decellularized murine lungs (Fig.4) are shown. Labeling of the entire porcine trachea and murine lungs was feasible. Consecutive imaging slices through the intact lungs revealed spatially resolved details that could be depicted because of full penetration of the labeling agent into the organ. Toxicity assays with HUVECs seeded on labeled scaffolds with concentrations up to 0.4mM exhibited no deleterious effects on cells grown for 24 and 72 hours (Fig.5). The mechanism of dECM labelling with MnPNH2 was investigated by transmission electron microscopy. dECM fibers deposited on carbon grids and labelled with 0.2 and 0.4mM MnPNH2 exhibited porphyrin binding and aggregation directly on dECM fibers but not anywhere else (data not shown). Furthermore, retention studies with dECM porcine and whole lung scaffolds revealed minimal release of MnPNH2 with daily changes under 1% by the fourth day and less than 20% total over 30 days.Discussion
Initial labelling optimizations demonstrated the ability to achieve substantial contrast enhancement of dECM scaffolds with T1 reductions of almost 10-fold. In-vivo and ex-vivo imaging confirmed that even at low doses of 0.2 mM, very bright contrast was achieved, suggesting that adequate signal-to-noise can be easily attained if further dose reduction were desired. Another important result is the ability of MnPNH2 to permeate throughout thick 3D tissues and thereby uniformly label whole dECM organs and not merely superficial layers. With high retention over a month period we are now equipped to potentially track dECM degradation longitudinally in vivo.Conclusion
We have reported on a new method and contrast agent to label and image dECM scaffolds of various forms and tissue types on MRI. Excellent sensitivity was achieved both in vitro and in vivo at 3 Tesla using labeling concentrations that had no negative effects on cell viability, proliferation, or metabolism. This study lays the foundation for long-term in-vivo monitoring in future dECM regeneration investigations.Acknowledgements
D.A.S. is funded by the Ontario Graduate Scholarship. T.K.W. is funded by Medicine by Design Cycle 1 Team Grant. H-L.M.C. is funded by the Canada Foundation for Innovation/Ontario Research Fund [grant #34038], a Translational Biology and Engineering Program Seed Grant, a Canada First Research Excellence Fund Medicine by Design Cycle 1 Team Grant, the Dean’s Spark Professorship, and the Natural Sciences and Engineering Research Council of Canada [grant #355795].References
1. Szulc, D. A. & Cheng, H. L. M. One-Step Labeling of Collagen Hydrogels with Polydopamine and Manganese Porphyrin for Non-Invasive Scaffold Tracking on Magnetic Resonance Imaging. Macromol. Biosci. 19, 1–12 (2019).
2. Brown, A. L. et al. 22 Week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials 23, 2179–2190 (2002).
3. Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S. & Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29, 1630–1637 (2008).
Figures