0622
Simultaneous Multi-Slice Radial Echo Volumar Imaging for Fast Simultaneous Multi-Parametric Imaging1Medicine, University of Hawaii, Honolulu, HI, United States, 2University of Hawaii, Honolulu, HI, United States, 3New York University, New York, NY, United States
Synopsis
Echo Planar Imaging (EPI) is one of the most widely used fast acquisitions and has been shown to be useful for high-resolution Simultaneous Multi-Parametric (SMP) imaging. However, obtaining high spatial resolutions requires k-space segmentation which has a high sensitivity to motion. Radial sampling is promising because of its continuous k-space center update which can be used for self-navigation and its suitability for undersampling because of benign artifacts. We describe a new k-t sampling strategy based on a Radial Echo Volumar Imaging method for fast SMP imaging. Whole brain images including susceptibility, B0 and T2* maps acquired at 3T are presented.
Introduction
Echo Planar Imaging (EPI) is one of the most widely used fast MRI acquisition methods (1). In particular simultaneous multi-slice (SMS) imaging with “Blipped-CAIPI” EPI using parallel imaging has largely contributed to the success of EPI for rapid imaging (2). The EPI technique has been shown to be useful for rapid high-resolution structural imaging and most recently for Simultaneous Multi-Parametric (SMP) imaging (3). However, obtaining high spatial resolutions requires k-space segmentation which has a high sensitivity to motion and other physiological effects (4). In the context of motion robust imaging radial sampling is particularly promising because of its continuous update of the k-space center which can be used for self-navigation purposes (5). Furthermore, radial sampling is also suitable for undersampling because of its benign aliasing artifacts that are streak-like in nature as opposed to image replicas common to Cartesian imaging. Here we describe a flexible and efficient SMS radial EPI k-t sampling strategy based on a Radial Echo Volumar Imaging (REVI) method in the context of fast SMP imaging. Whole brain 1x1x2mm3 structural images including susceptibility, B0 and T2* maps acquired in two minutes at 3T are presented.Trajectory Design and Data Sampling
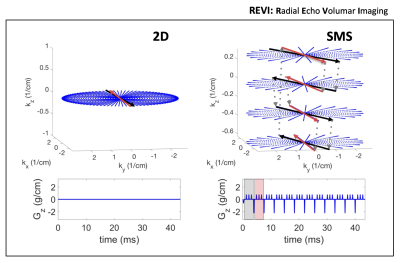
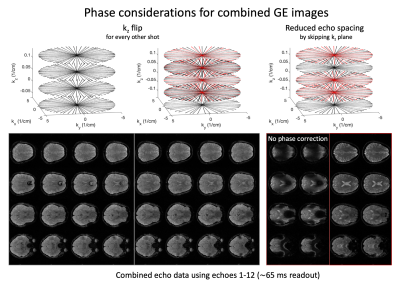
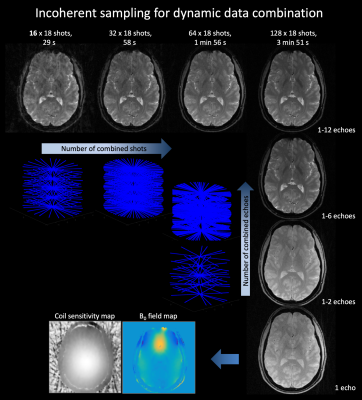
In radial EPI multiple radial k-space lines are traversed in a gradient echo acquisition to cover large sections of the kxy plane in a single shot (Figure 1) (6). Due to the limitations of gradient performance a single excitation is usually not sufficient to sample kxy at Nyquist for high resolution imaging. Therefore, to enhance sampling density multi shot segmentation can be implemented by rotating the radial EPI readout trains of consecutive excitations by an arbitrary angle (φshot). SMS REVI is based on this sampling design but further incorporates z gradient blips in addition to the gradient blips to rotate between spokes (φecho). After sampling a line in each of the N SMS kz planes a rewinder blip returns sampling to the initial kz plane. With the SMS REVI trajectory multiple lines in SMS k-t space can be sampled effectively within a single shot. To alter the sequence of kz sampling the kz blipping can be flipped for consecutive shots. Furthermore, kz planes can be left out/skipped to decrease echo spacing (Figure 2). The combination of golden angle rotations between shots (φshot≈111.25°) and "tiny" golden angle rotations between echoes (φecho≈49.75°) enable flexible reordering schemes of different echo data and shot-to-shot data combinations (Figure 3). A 65 ms SMS REVI trajectory with NSMS=4 readout can give rise to 16 echoes (total of 39 lines).Methods
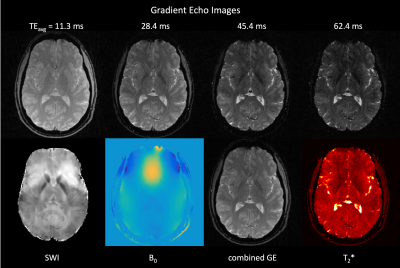
Acquisition: Human brain MRI data were acquired on a Siemens 3T Prisma scanner using a spoiled SMS gradient echo sequence (NSlicegroup=18, NSMS=4, 20° flip angle, TR=1800 ms and TEs=4.9, 9.2, 13.5, 17.7, 22.0, 26.2, 30.5, 34.8, 39.0, 43.3, 47.5, 51.8, 56.1, 60.3, 64.6, 68.8 ms). Scans of 128 shots were acquired and retrospectively undersampled for whole brain imaging (FOVxy=210 mm, FOVz=144 mm). The REVI trajectory was rotated by the golden angle (φshot≈111.25°) and kz gradients were flipped between consecutive excitations (Figure 2). Reconstruction: An iterative reconstruction modeling coil sensitivities (SENSE) and B0 field inhomogeneities within the signal equation was used to obtain 72 slices at a resolution 1x1x2mm3. B0 field maps (via regularized field map estimation (7)) and coil sensitivity maps (via adaptive array-combination (8)) were obtained directly from the SMS REVI data itself. Non-uniform fast Fourier transform (NUFFT) on k-space data was performed using the MIRL toolbox (https://web.eecs.umich.edu/~fessler/code/) in combination with a limited-memory BFGS Poblano toolbox (http://github.com/sandialabs/poblano_toolbox) algorithm for minimization. All reconstructions were performed offline using Matlab. T2* maps were obtained by fitting gradient echo data to a mono-exponential model. Quantitative susceptibility maps were generated using the MEDI toolbox (http://pre.weill.cornell.edu/mri/pages/qsm.html).Results
SMS REVI presents an efficient and versatile sampling strategy for fast time-resolved imaging (Figure 1). A general challenge with radial EPI is that the long readouts which continuously cross the center of k-space lead to severe off-resonance artifacts. In combination with the model-based reconstruction, which accounts for B0 field inhomogeneities, high quality images with only minor signal dropout and distortion can be obtained (as shown by the images in Figure 2). Rotation of the trajectory by the golden angle between excitations and between echoes permits for a continuous and unique k-space sampling through time such that images can be reconstructed with variable temporal resolution (Figure 3). High quality whole brain (72 slices with 1x1x2mm3 resolution) T2* and susceptibility maps could be obtained from a two minute acquisition (Figure 4).Discussion
The results suggest that SMS REVI is a very promising approach to fast SMP imaging. The use of intrinsic B0 maps in the model based reconstruction greatly reduces off-resonance artifacts common to radial EPI. The dual golden angle approach presents a versatile sampling strategy with great potential for self-navigated motion correction. The extension to other parameters such T1 and other modalities such as free breathing body imaging are also very promising and significant.Acknowledgements
This project was supported by the NIH grant R01DA019912References
(1) Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys C 1977;10:L55-L58.
(2) Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 2012;67(5):1210-1224.
(3) Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med 2019;81(6):3599-3615.
(4) Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging 2015;42(4):887-901.
(5) Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med 2017;78(2):565-576.
(6) Silva AC, Barbier EL, Lowe IJ, Koretsky AP. Radial echo-planar imaging. J Magn Reson 1998;135(1):242-247.
(7) Funai AK, Fessler JA, Yeo DT, Olafsson VT, Noll DC. Regularized field map estimation in MRI. IEEE Trans Med Imaging 2008;27(10):1484-1494.
(8) Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med 2000;43(5):682-690.
Figures