0621
Rapid volumetric 3D MRI via simultaneous-multi-slab, multi-echo spatiotemporal encoding (SMS-ME SPEN)1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2College of Electronic science and technology, Xiamen University, Xiamen, China, 3Centre de RMN à Très Haut Champs, Lyon, France, 4Massachusetts General Hospital, Boston, MA, United States
Synopsis
SPatiotemporal ENcoding (SPEN) is a 2D single-shot MRI method with higher immunity to artifacts than EPI-based counterparts. The present study extends SPEN scans to 3D volumetric measurements, to achieve imaging over a 3rd dimension at higher resolution in minimal acquisition times. simultaneous multi-slab (SMS) and multi-echo (ME) kz-encoding procedures are here combined to cope with the SAR complications that would ensue from simply repeating 2D acquisitions over multiple slices. A framework to appropriately reconstruct and process 3D SMS-ME SPEN data to ensure the image quality by taking motion artifacts derived from different dimensions into account is also proposed, and demonstrated.
Introduction
SPatiotemporal ENcoding (SPEN)1 is a scanning technique that relies on frequency swept pulses combined with readout gradients to collect 2D images of a sample in a single scan. SPEN is subject to fewer distortions than EPI — at the expense of a higher SAR (power deposition) associated to its use of frequency swept RF pulses,2 and of slightly longer echo times when implemented under full-refocusing conditions providing additional immunity by refocusing T2* effects throughout the data acquisition.3 The present study extends SPEN scans to 3D volumetric measurements, by combining simultaneous multislab (SMS) and multi-kz encoding procedures to achieve higher resolution images along the 3rd dimension, while coping with the SAR complications that ensue from simply repeating 2D acquisitions over multiple slices.Method
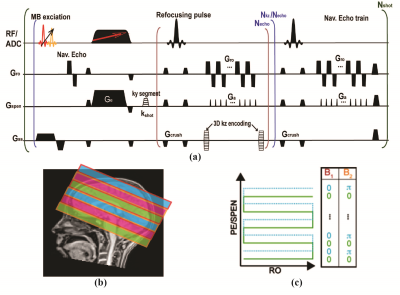
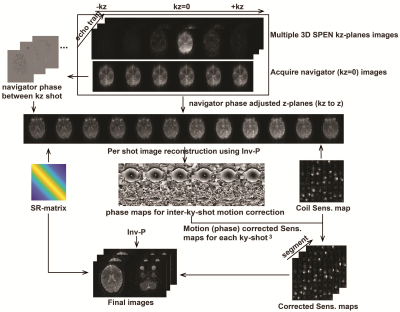
The pulse sequence here introduced is shown in Fig. 1a. Its SMS pulse excites multiple z-slabs; these slabs are additionally encoded by a CAIPIRINHA-like4 procedure (Fig. 1c) that improves the efficiency of an unaided parallel image reconstruction. Sensitivity maps ae acquired in separated, low-resolution single-band SPEN scans. To resolve multiple slices within these multi-band z-slabs, a multi-echo delivering single-shot full 2D trains possessing different kz values within each slab, is also implemented (Fig. 1a). A low-resolution kz = 0 (navigator) image is acquired immediately following the acquisition of these multi-echo trains, for every scan, in order to reduce motional effects between kz encodings. Interleaved SPEN acquisitions 5 are used if in-plane image resolution needs to be improved. Figure 2 shows a reconstruction scheme customized to this sequence, which follows: 1) Generation of shot-to-shot phase variation maps from the navigator data, used to remove bulk movement throughout the kz-sampling. 2) A FFT along the corrected kz dimension. 3) An inverse operation (Inv-P) based on the L2 conjugate gradient approach in the ESPIRiT algorithm, 6-9 combining SPEN’s super-resolution (SR) matrix and the coils’ sensitivity maps to deliver an uncorrected 2D SPEN image set. 4) Calculation of each band’s phase independently for every ky- and diffusion-weighted scan using Inv-P. 5) Multiplication of the phase maps from step 4) by sensitivity maps to adjust each shot’s data. 5) Reconstructing the data from step 5) using Inv-P.Results & Discussion
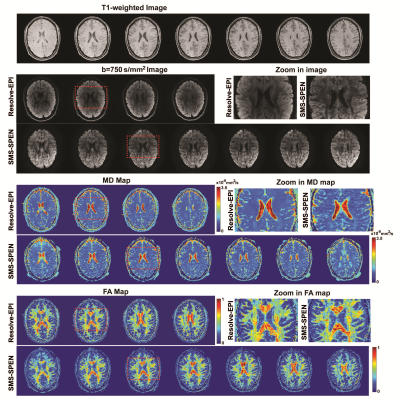
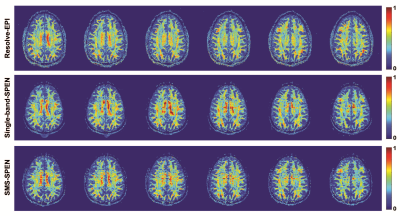
DTI experiments with 20 diffusion directions were conducted on a human volunteer at 3T on a Siemens Prisma MRI scanner using 32 channels head coil, following suitable written consent. 2D RESOLVE-based10 EPI images were acquired as a reference with 75% partial Fourier sampling in ky dimension and GRAPPA 2x acceleration. For the 3D SMS-ME SPEN tests dual band pulses were used, each one including two kz echoes (plus a navigator echo) collected for a total of 12 kz values. These were collected within 6 scans for two slab simultaneously, while at the same time interleaving 6 segments in these scans along the ky (SPEN) dimension. Processing used the CAIPIRINHA scheme in Figure 1c and pipeline in Fig. 2. Both kz encodings and navigator images were acquired with 75% partial Fourier sampling along kx. Figure 3 compares 3D SMS-SPEN, RESOLVE -EPI and 3D T1-FLAIR (0.86 x 0.86 x1.25mm) brain results on a human volunteer. In the 3D SMS-ME SPEN resolution=1.15 x1.30 x1.25mm, acqusition bandwidth (BW) = 7.7kHz, TE=80ms, TR=1.3s, total scan time=16mins. In the RESOLVE-EPI resolution=2.0 x2.0x 2.0mm, BW= 3.1kHz,TE=40ms, TR=6 s, total scan time=11mins. Notice that this 2x2x2mm resolution is the best that the state-of-the-art without signal averaging, scanner-supplied 2D RESOLVE-based DTI sequence can reach. It follows that 3D SMS-ME SPEN can reach higher resolution DTI images with comparable SNR; although their acquisition times are a bit longer, motion correction along ky and kz makes SPEN imaging tolerant to patient motions, boding well for more challenging areas. Figure 4 compares 3D SMS-ME SPEN DTI images against 3D counterparts collected with ME but a single band. RESOLVE-EPI data with 3 times averaging (resolution=1.3 x1.3 x 1.3mm, BW= 3.1kHz,TE=40ms, TR=4s, total scan time=20mins) is also presented. While the single-band and the 3D SMS SPEN data have the same resolution without signal averaging (1.25 x1.25 x1.25mm, BW= 7.7kHz,TE=71ms, TR=1.3s, two kz echoes in one shot), the total scan time was 16 mins for the SMS SPEN and 30 mins for the single-band SPEN. 3D SMS SPEN thus delivers comparable image quality as RESOLVE and single-band SPEN counterparts, but required shorter acquisition times than these other sequences.Conclusion
3D SMS-ME SPEN can acquire high-resolution images in a clinical 3T MRI scanner with sufficient SNR and tolerance to image distortion, to become a useful aid in diffusion determinations. SMS, multi-echo CAIPIRINHA and interleaving procedures help reducing the acquisition time to a minimum, while providing high robustness, good sensitivity, good separation and cross-talk suppression among the simultaneously excited z-slabs, while only a slight increase in the SAR value. This work also introduced a suitable framework to appropriately reconstruct and process these 3D SMS-ME SPEN data, that we envision will serve well in mapping ADC and in DWI for other, more challenging regions than the brain.Acknowledgements
This work was funded by the Israel Science Foundation (grants 2508/17 and 965/18), by the Kimmel Institute of Magnetic Resonance (Weizmann Institute), by China Scholarship Council (CSC) grant 201806310085, and by Israel’s Planning and Budget Committee (Lingceng Ma, international student fellowship).References
(1) . Tal A, Frydman L, 2006. Spatial encoding and the single-scan acquisition of high definition MR images in inhomogeneous fields. Journal of Magnetic Resonance. 2006; 182:179–194.
(2) Liberman G, Solomon E, Lustig M, et al. Multiple‐coil k‐space interpolation enhances resolution in single‐shot spatiotemporal MRI. Magnetic Resonance in Medicine. 2018; 79(2): 796-805.
(3) Schmidt, R., Frydman, L. New spatiotemporal approaches for fully refocused, multislice ultrafast 2D MRI. Magnetic Resonance in Medicine. 2014; 71:711–722.
(4) Breuer FA, Blaimer M, Heidemann RM, et al, Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) for Multi-Slice Imaging. Magnetic Resonance in Medicine. 2005; 53:684–691.
(5) Seginer A, Schmidt R, Leftin A, et al. Referenceless reconstruction of spatiotemporally encoded imaging data: Principles and applications to real-time MRI, Magnetic Resonance in Medicine. 2014; 72: 1687-1695.
(6) Cousin SF, Liberman G, Solomon E, et al. A regularized reconstruction pipeline for high‐definition diffusion MRI in challenging regions incorporating a per‐shot image correction. Magnetic Resonance in Medicine. 2019; 82(4):1322-1330.
(7) Tamir JI, Ong F, Cheng JY, Uecker M, Lustig M. Generalized magnetic resonance image reconstruction using the Berkeley Advanced Reconstruction Toolbox. In ISMRM Workshop on Data Sampling & Image Reconstruction, Sedona, AZ, 2016.
(8) Uecker M, Ong F, Tamir JI, et al. Berkeley advanced reconstruction toolbox. In Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Ontario, Canada, 2015. p. 2486.
(9) Uecker M, Lai P, Murphy MJ, et al. ESPIRiT–an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magnetic Resonance in Medicine. 2014;71:990–1001.
(10) Holdsworth SJ, Skare S, Newbould RD, et al. Readout-segmented EPI for rapid high resolution diffusion imaging at 3T. European Journal of Radiology. 2008; 65:36–46.
Figures