0616
Spiral Crisscrossing Echo Planar Time-resolved Imaging (SCEPTI)1A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
A new technique, termed Spiral Echo Planar Time-resolved Imaging (SKEPTIC), was developed to address both EPI’s geometric distortion and blurring and augment the recently introduced Echo Planar Time-resolved Imaging (EPTI). In SKEPTIC, the (2+1)-D k-t space is traversed using several matching out-in spirals within a single shot, and can benefit from additional rotated and time-jittered shots. The out-in multi-spiral trajectory is incoherent with field inhomogeneity phase evolution in both axes. This results in the ability to deliver single-shot 1.9mm2 in-plane resolution distortion-less, sharp multi-echo images with B0 and T2* mapping, and inherent motion, phase and B0-variation estimates for multi-shot imaging.
Introduction
Echo planar imaging (EPI) delivers fast imaging but suffers from B0-inhomogeneity induced geometric distortion and T2*-decay induced image blurring along the phase encoding direction. These issues were tackled in the recently introduced multi-shot EPI approach termed EPTI[1]. EPTI encodes the full k-t space of the acquisition efficiently using a highly-undersampled spatio-temporal CAIPI trajectory, to recover distortion-free, sharp multi-contrast images, along with the underlying $$$B_0$$$ and $$$T_2^*$$$ map, in a few EPTI-shots. Other alternatives for distortion correction include using an additional acquisition with opposing phase-encoding[2], or including acquisition of shots with opposing PE direction in the acquisition scheme[3].Spiral acquisition benefits from more efficient k-space transversal with a faster encoding when compared to EPI. However the $$$B_0$$$-inhomogeneity induced phase evolution affects the reconstructed image in both spatial dimensions (x&y) in spiral trajectories, and greater care needs to be taken into signal modelling to produce a high-quality reconstruction. Thus, inhomogeneity correction techniques and the combined estimation of the image along with field inhomogeneity and T2* map have been suggested early on[4,5]. Moreover, emerging sub-space reconstruction[6] has advanced the capabilities of a number of MR acquisitions, including to increase EPTI’s SNR and acceleration capability[7], and should also benefit spiral acquisitions.
In this study, a new technique termed Spiral Crisscrossing Echo Planar Time-resolved Imaging (SCEPTI) was developed for efficient time-resolved multi-contrast imaging. SCEPTI utilizes highly-accelerated ‘θ-t CAIPI’ out-in spirals to efficiently fill out k-t space along with subspace reconstruction to provide rapid distortion-free multi-contrast imaging.
Methods
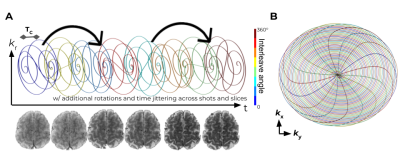
Aiming to efficiently cover k-t space, the SCEPTI trajectory (Fig. 1) is built on a series of out-in spirals, each one termed an “inner-shot”. Within each inner-shot, at the end of the out-spiral the trajectory crosses to the mirrored (-kx,-ky) location and continues with a mirrored in-spiral, such that the k-space samplings of the out- and the in-spirals complement each other as per multi-shot spiral. The subsequent inner-shots are rotated based on discrete golden-angle reasoning- maintaining a balanced coverage of k-space along portions of the acquisition time. Subsequent SCEPTI-shots repeat this structure with rotation and time-jittering to further fill k-t space in an efficient manner. Additionally, the trajectory is rotated between slices, to create a “z-θ CAIPI” stack-of-spirals structure where stacking is along inner-shots, shots, and z (rather than kz). This acts to improve incoherency of aliasing artifacts along the slice dimension which facilitates joint reconstruction across slices with e.g. TV constrain.Two different 3-shot SCEPTI trajectories were designed for 2mm in-plane acquisition based on varying the out/in-spiral duration $$$T_C$$$, while keeping within the PNS limits calculated using the SAFE[8] model. The ability of these trajectories in providing high-quality time-resolved imaging was compared through simulations based on a gold-standard fully-sampled k-t dataset from a lengthy multi-echo GRE scan (32-channel, 3T, echo-spacing 0.7ms, 70 T2*w-echoes). Point-spread-function analysis was also performed.
Based on these analyses, the in-vivo acquisition was performed. SCEPTI acquisition with a 48ms gradient-echo readout duration, 8 inner-shots with $$$T_C$$$ of 3ms, 2x2x1mm3.
All reconstructions were performed using the subspace EPTI approach[7]. Here TV constraint is added along z to take advantage of z-θ CAIPI sampling across slices.
Results
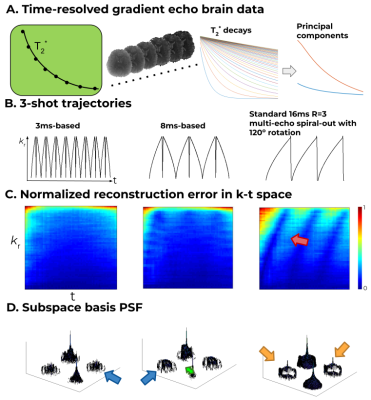
Fig. 2 shows the subspace reconstruction comparison on simulated data between the two different SCEPTI trajectories for 2 mm brain imaging (with $$$T_C$$$ of 3 and 8ms), as well as comparison to standard multi-echo spiral acquisition at Rinplane=3. Normalized reconstruction errors with respect to gold-standard fully-sampled GRE are shown across k-t space. In a multi-shot spiral, high localized error regions (red arrow) are present resulting from sampling gaps in k-t space, while the two SCEPTI trajectories provide similarly high-quality performance. Additional analysis of the spatiotemporal reconstruction performance of these SCEPTI trajectories is performed using point-spread-function analysis across the subspace coefficient basis[9]. With this analysis, strong cross talks are observed in the multi-shot spiral case (orange arrows), which are reduced with SCEPTI trajectories. A trade-off in spatial vs temporal resolution between the SCEPTI trajectories cases can be observed.The Tc3ms trajectory contains the lowest cross talk (blue arrows) which points to high temporal specificity, while the Tc8ms trajectory contains sharper passthrough PSF term (green arrow) with lower sidelobe pointing to high spatial specificity.
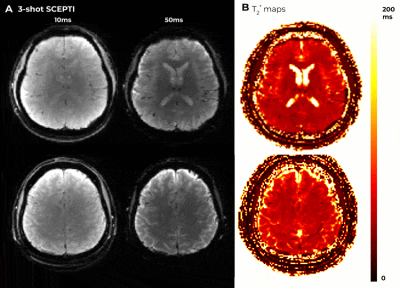
The 3-shot Tc3ms-SCEPTIC was utilized in in-vivo acquisitions. Fig. 3 shows high quality time-resolved T2w images and T2* map across representative slices.
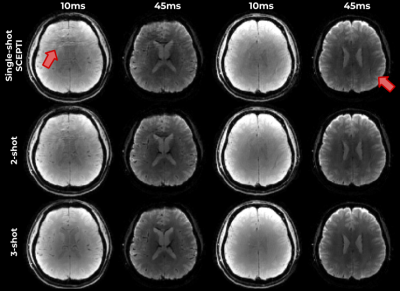
Fig. 4 shows reconstructions performed using 1, 2 and 3 shots of SCEPTI data, where reconstruction performance remains relatively good with some minor artifacts in the rapid 1 shot case with data from just 48ms of acquisition.
Discussion and Conclusion
We introduce a spiral echo-planar time-interleaved sequence that delivers multi-echo distortion-free images and rapid parametric mapping.Moreover, we demonstrate that reasonable reconstruction can be obtained in just a single shot of SCEPTIC, offering a potential for higher temporal resolution as well as the ability to better track motion and phase corruptions across shots and incorporate such information to perform better reconstruction jointly across shots for high-quality reconstruction.
The spiral-based SCEPTI trajectory design is currently PNS limited, with hardware Gmax and slew not being fully exploited. Emerging work on gradient design with better PNS tradeoff could further improve SCEPTI encoding and enable higher acceleration capabilities.
Acknowledgements
This work was supported in part by NIH research grants: NIH R01EB019437, R01EB020613, R01 MH116173, and U01EB025162.References
1. Wang, F., Dong, Z., Reese, T.G., Bilgic, B., Katherine Manhard, M., Chen, J., Polimeni, J.R., Wald, L.L. and Setsompop, K., 2019. Echo planar time‐resolved imaging (EPTI). Magnetic resonance in medicine, 81(6), pp.3599-3615.
2. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003;20:870-888.
3. Zahneisen B, Aksoy M, Maclaren J, Wuerslin C, Bammer R. Extended hybrid-space SENSE for EPI: Off-resonance and eddy current corrected joint interleaved blip-up/down reconstruction. NeuroImage 2017;153:97-108.
4. Sutton, B.P., Noll, D.C. and Fessler, J.A., 2003. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE transactions on medical imaging, 22(2), pp.178-188.
5. Sutton, Peltier, Fessler, Noll, Simultaneous estimation of I0, R2*, and field map using a multi-echo spiral acquisition, Proc. ISMRM 2002
6. Liang Z. Liang, Z.P., 2007, April. Spatiotemporal imagingwith partially separable functions. In 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro (pp. 988-991). IEEE.
7. Dong Z, Wang F, Reese TG, Bilgic B, Setsompop K. Echo Planar Time-Resolved Imaging (EPTI) with subspace constraint and optimized kt trajectory. In: Proc Intl Soc Mag Reson Med. ; 2019.
8. Hebrank, F.X. and Gebhardt, M., 2000. SAFE model—a new method for predicting peripheral nerve stimulation in MRI. In Proc Intl Soc Mag Res Med (Vol. 8, p. 2007).
9. Tamir, J.I., Uecker, M., Chen, W., Lai, P., Alley, M.T., Vasanawala, S.S. and Lustig, M., 2017. T2 shuffling: sharp, multicontrast, volumetric fast spin‐echo imaging. Magnetic resonance in medicine, 77(1), pp.180-195.
Figures