0615
Sub-millisecond 2D MRI of the Vocal Fold Oscillation using Single Point Imaging with Rapid Encoding (SPIRE)1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research Freiburg Site, German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Division of Phoniatrics and Pediatric Audiology, Department of Otorhinolaryngology, Head and Neck Surgery, Ludwig-Maximilians-University, Munich, Germany, 4Institute of Musicians' Medicine, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
We use single point imaging with rapid encoding (SPIRE) to image the vocal fold oscillations in the coronal plane. SPIRE is able to image fast, repetitive and two-dimensional motion, because the temporal resolution does not depend on TR but on the duration of the fast-switching phase encoding gradients, which is below one millisecond in this work. Data are gated using electroglottography and projection navigators are acquired during the sequence to detect shifts in larynx position which is corrected during reconstruction.
Introduction
Recently, MRI was applied for the first time to dynamically image the rapid oscillation of the vocal folds using very short phase encoding gradients along the 1D direction of vocal fold motion1. Vocal fold MRI is less invasive and offers soft tissue contrasts, but the transverse view, under which the vocal fold motion is observed, is the same as in laryngeal stroboscopy – the gold standard in dynamic vocal fold imaging. Clinically, a coronal view of the larynx would be more interesting as it could show how the vocal folds move towards one another (RL-direction) and get pushed upward by the pressure from the lung (SI-direction) forming the mucosal wave2. However, this 2D motion cannot be encoded as described in1, but requires fast phase encoding gradients along both directions of motion. In this work we therefore propose a 2D single point imaging with rapid encoding (SPIRE) technique for dynamic imaging of the vocal fold oscillation which employs navigator techniques for combination of data from separate phonation periods.Methods and Materials
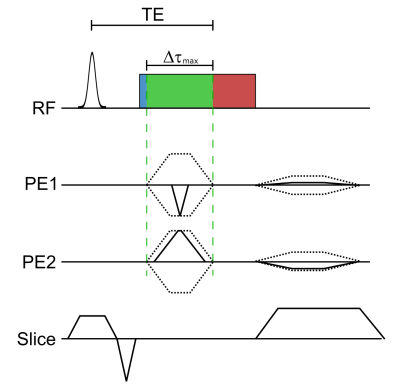
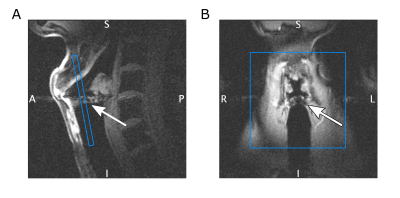
A diagram of the SPIRE sequence is shown in Figure 1. After slice selection the ADC is switched on immediately, and 20 µs later the phase encoding (PE) gradients are applied. The PE gradients are applied as short as possible ($$$\Delta\tau_\mathrm{max}$$$ = 640 µs, compromising between peripheral nerve stimulation and speed), so that phase encoded k-space points closer to the center have a shorter temporal resolution. To achieve a resolution below 1 mm, a field of view of 70 mm was chosen, and the k-space matrix of 80x80 was 4x undersampled using a Poisson disc scheme to reduce measurement time. With TE = 2.16 ms, TR = 4.49 ms and 20 repetitions, the total acquisition time was 2 min 17 s for a single 2D image. To image the vocal fold oscillations, a volunteer was instructed to sing continuously during the SPIRE measurement and to interrupt singing for breathing whenever necessary. The volunteer was hearing the desired phonation frequency of 146 Hz (D3) over the head phones, and an electroglottogramm (EGG) was acquired during the measurement to gate the MR data. The EGG system comprises two MR-safe electrodes3 which are attached to the left and right of the larynx, and provides a signal which changes with the contact area of the vocal folds. For signal reception a loop coil was placed at the level of the vocal folds on top of the electrodes. Slice positioning can be seen in Figure 2. To synchronize EGG and MR data, an optical trigger signal was sent from the MR system and recorded by the EGG. For image reconstruction, the center of each PE gradient was identified in the EGG data and a sinusoidal function is fitted to extract the motion phase of the vocal folds oscillation at that time. k-Space data were sorted into 7 different dynamic frames according to their motion phase. The resulting 80x80x7 k-space was reconstructed using compressed sensing4 and total variation regularization in the spatial and temporal domain respectively using BART5. EGG data was also used to detect breathing and reject the compromised MR data.Repeated breathing and sustained phonation causes translational motion of the larynx, which has a detrimental impact on image quality. In this study, additional navigator projections along the SI direction were used to compensate for breathing motion. Therefore, navigator data were acquired during the SPIRE measurement, and compared with a reference navigator using a phase only cross correlation (POCC) algorithm6. The shift in SI direction was determined every 500th TR (i.e., every 2.2 s), and was linearly interpolated in time between the navigators to correct for displacements.
Results
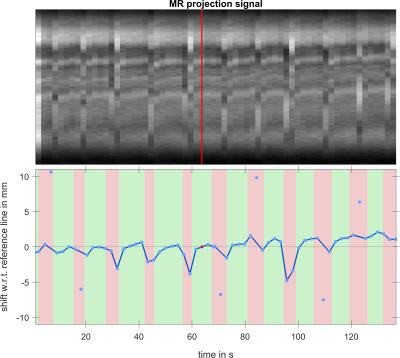
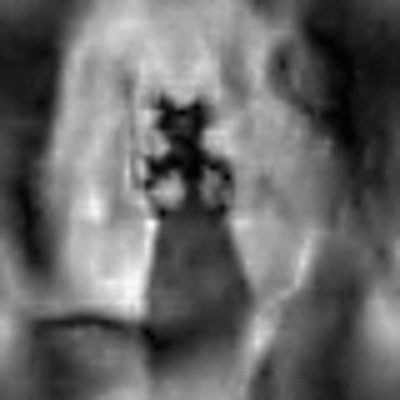
In Figure 3 the navigator signal and the calculated displacement shift with respect to a reference navigator are displayed. As can be seen, shifts of up to +/-11 mm in SI direction over the whole measurement are calculated. Two motion patterns emerge from the data, a rise of the vocal fold position during one exhalation (vibrato) and an additional upward drift over the whole measurement. The reconstructed vocal fold oscillation is shown in Figure 4 as an animation. While motion along both encoding axes is visible, a full closure of the glottal gap between left and right vocal fold cannot be seen.Discussion
In this pilot study, for the first time 2D MR images of the oscillating vocal folds are presented. With the current contrast the contact between the left and the right vocal fold could not be displayed because both the spatial resolution is very limited, and the different anatomical layers of the vocal folds give off very different signals. The use of navigators allows to partially compensate for position differences between phonation cycles, but additional position measurements are required to assess the precision of this compensation techniques. In general, the POCC algorithm can correct inplane movement, but is not suitable for out-of-slice motion, which might occur when larynx motion and slice orientation are not parallel. Future work will focus on reproducible positioning of the volunteers larynx and improvements in navigator signal to better preclude and correct subject motion. Additionally, prior knowledge about the surrounding tissue might improve image reconstruction.Acknowledgements
No acknowledgement found.References
[1] J. Fischer, T. Abels, A. C. Özen, M. Echternach, B. Richter, und M. Bock, „Magnetic resonance imaging of the vocal fold oscillations with sub-millisecond temporal resolution“, Magnetic Resonance in Medicine, Bd. 83, Nr. 2, S. 403–411, 2020.
[2] C. R. Krausert, A. E. Olszewski, L. N. Taylor, J. S. McMurray, S. H. Dailey, und J. J. Jiang, „Mucosal Wave Measurement and Visualization Techniques“, Journal of Voice, Bd. 25, Nr. 4, S. 395–405, Juli 2011.
[3] A. C. Özen u. a., „Ensuring safety and functionality of electroglottography measurements during dynamic pulmonary MRI“, Magnetic Resonance in Medicine, Bd. 76, Nr. 5, S. 1629–1635, Nov. 2016.
[4] M. Lustig, D. Donoho, und J. M. Pauly, „Sparse MRI: The application of compressed sensing for rapid MR imaging“, Magnetic Resonance in Medicine, Bd. 58, Nr. 6, S. 1182–1195, Dez. 2007.
[5] M. Uecker u. a., „Berkeley Advanced Reconstruction Toolbox“, in In Proc. Intl. Soc. Mag. Reson. Med., Toronto, 2015, Bd. 23, S. 2486.
[6] A. de Oliveira, J. Rauschenberg, D. Beyersdorff, W. Semmler, und M. Bock, „Automatic passive tracking of an endorectal prostate biopsy device using phase-only cross-correlation“, Magnetic Resonance in Medicine, Bd. 59, Nr. 5, S. 1043–1050, Mai 2008.
Figures