0606
Robust Morphological Myelin Imaging Using a Short TR Adiabatic Inversion Recovery Prepared Ultrashort Echo Time (STAIR-UTE) Sequence1UC San Diego, San Diego, CA, United States, 2VA health system, San Diego, CA, United States
Synopsis
To image myelin in brain more robustly on clinical scanners, we propose a Short TR Adiabatic Inversion Recovery prepared UTE (STAIR-UTE) sequence for volumetric myelin imaging in vivo. With STAIR technique, long T2 tissues with a broad range of T1s can be sufficiently suppressed. High myelin contrast can be robustly obtained with a TR less than 250 ms. The resultant myelin imaging shows a clear loss of myelin signal in multiple sclerosis (MS) lesions.
Introduction
Ultrashort echo time (UTE) sequences with echo times less than 50 µs can directly detect signals from myelin protons (1-4). However, over 90% of the UTE signal originates from long T2 water protons, even in myelin-rich white matter. Thus, sufficient long T2 suppression is crucial for direct myelin imaging. One commonly used approach is to use an adiabatic full passage (AFP) pulse to invert the longitudinal magnetizations of long T2 tissues, and UTE data is acquired at an appropriate inversion time (TI) with long T2 signal nulled (4-6). Thus, long T2 suppression is sensitive to the selection of TI in current IR-UTE techniques (3-7). A slight TI offset may cause significant long T2 contamination, as the residual long T2 signals may be higher than that of myelin. Moreover, T1 relaxation may change with age and pathology in the brain (8,9). This makes the corresponding TI determination more challenging. Furthermore, a single TI may not be sufficient to null all long T2 components due to T1 variations in different white matter regions in the same brain (10-11). To address the aforementioned problems in myelin imaging, we propose a Short TR Adiabatic Inversion Recovery prepared UTE (STAIR-UTE) technique for more robust long T2 suppression regardless of T1 variations in white matter.Methods
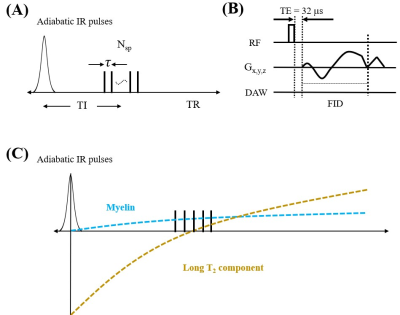
Figure 1A shows features of the 3D STAIR-UTE sequence (12,13). Following an AFP pulse, Nsp separate k-space spokes with an identical time interval, τ, are used for fast data acquisition (Figure 1B). Figure 1C shows the contrast mechanism of myelin imaging. The longitudinal magnetizations of long T2 components are inverted by the AFP pulse and start to recover right after the AFP pulse. In contrast, the longitudinal magnetization of myelin is not inverted, but largely saturated, due to the fast T2 relaxation of myelin protons during the relatively long AFP pulse (14,15). Thus, the myelin magnetization recovers from zero, and pure myelin signals can be acquired at a specific TI when the long T2 components are nulled.Numerical simulation was performed to investigate the efficiency of long T2 suppression for the STAIR scheme with different TRs. The simulated T1 values of the long T2 components ranged from 20 to 2500 ms, which cover T1 values for both white matter and gray matter. The TIs were determined by Eq. [8] in Ma et al. (13) in order to minimize the average signals from the long T2 components with T1 values ranging from 500 to 1500 ms. The Sratio is defined as the signal intensity ratio between myelin and long T2s.
A series of water phantoms with different T1s were prepared. Nine 5-mL water phantoms were prepared with MnCl2˙4H2O concentrations of 0.0055, 0.01, 0.015, 0.0195, 0.0265, 0.0375, 0.085, 0.18, and 1.4828 g/L. The nine phantoms were then placed in parallel in a cylinder container filled with 1% agarose. These phantoms were scanned using the STAIR-UTE sequence with different TRs of 50, 100, 150, 200, 250, 500, and 800 ms. Other sequence parameters were as follows: field of view (FOV)=15×15×16 cm3, acquisition matrix=160×160×40, TE=32 μs, Nsp= 5, τ=5 ms, flip angle=20°, and bandwidth=125 kHz.
In vivo brain imaging was performed on ten healthy volunteers (19-45 years of age, 6 males and 4 females) and ten patients with multiple sclerosis (MS) (35-70 years of age, 3 males and 7 females). Informed consent was obtained from all subjects in accordance with guidelines of the institutional review board. The sequence parameters were as follows: FOV=22×22×30 cm3, matrix=140×140×60, TR/TI=140/61 ms, TE=32 μs, Nsp=5, τ=3.2 ms, flip angle=32°, bandwidth=125 kHz, oversampling factor=2.3, scan time=9.5 min. Clinical T2-FLAIR sequence was used for comparison.
Results and Discussion
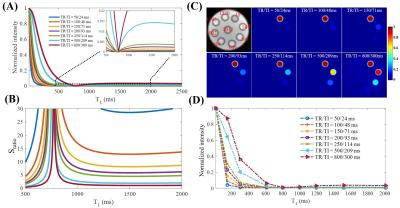
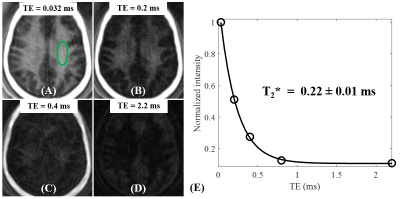
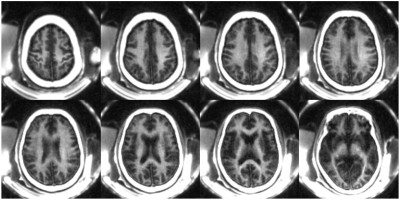
The numerical simulation study suggests that signal suppression for long T2 tissues with different T1s using the conventional IR scheme with a relatively long TR (e.g. TR = 800 ms) is sensitive to the selection of TI (Figure 2A). Additional efforts are needed to determine the optimal TI for nulling specific long T2 components. In comparison, the proposed STAIR scheme with TR<250 ms can effectively suppress long T2 tissues with a broad range of T1s with a single TR/TI combination and a high myelin contrast can always be obtained (Figure 2B). The phantom study further demonstrated the sequence’s ability to efficiently suppress long T2 signals with a wide range of T1s (Figures 2C and 2D).The volunteer study demonstrated the robustness of the STAIR-UTE sequence in efficient suppression of long T2 components in white matter and selective imaging of myelin, as confirmed by the short T2* of 0.22±0.01 ms (Figure 3). The representative whole brain myelin images acquired with the 3D STAIR-UTE sequence from the same volunteer are shown in Figure 4. Figure 5 shows comparisons of STAIR-UTE and clinical T2-FLAIR images for three representative patients with MS. Hyperintense lesions detected in T2-FLAIR images show as signal loss in corresponding STAIR-UTE images, which demonstrates short T2 myelin signal loss and, thus, demyelination of MS lesions.
Conclusion
The 3D STAIR-UTE sequence with a short TR/TI combination provides robust suppression of long T2 components in white matter, allowing selective volumetric imaging of myelin in white matter of the brain in vivo.Acknowledgements
The authors acknowledge grant support from NIH (1R01NS092650) and VA Clinical Science and Rehabilitation R&D Awards (I01CX001388 and I01RX002604).References
1. Waldman A, Rees JH, Brock CS, Robson MD, Gatehouse PD, Bydder GM. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology 2003; 45:887–892.
2. Horch AR, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med 2011; 66:24–31.
3. Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci USA 2012; 109:9605–9610.
4. Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage 2014;15;87:32-41.
5. Sheth V, Shao H , Chen J, Vandenberg S, Corey-Bloom J. Bydder GM, Du J. Magnetic resonance imaging of myelin using ultrashort Echo time (UTE) pulse sequences: phantom, specimen, volunteer and multiple sclerosis patient studies. Neuroimage 2016; 136, 37–44.
6. Seifert AC, Li C, Wilhelm MJ, Wehrli SL, Wehrli FW. Towards quantification of myelin by solid-state MRI of the lipid matrix protons. NeuroImage 2017;163:358-67.
7. Fan S-J, Ma Y, Zhu Y, et al. Yet more evidence that myelin protons can be directly imaged with UTE sequences on a clinical 3T scanner: Bicomponent T2* analysis of native and deuterated ovine brain specimens. Magn. Reson. Med. 2018;80:538–547
8. Suzuki S, Sakai O, Jara H. Combined volumetric T1, T2 and secular-T2 quantitative MRI of the brain: age-related global changes (preliminary results). Magn. Reson. Imaging 2006;24:877–887.
9. Margaret Cheng H-L, Stikov N, Ghugre NR, Wright GA. Practical medical applications of quantitative MR relaxometry. J. Magn. Reson. Imaging 2012;36:805–824.
10. Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: searching for common ground. Magn. Reson. Med. 2015;73:514–522.
11. Rooney WD, Johnson G, Li X, et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 2007;57:308–318.
12. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn. Reson. Med. 2016;76:577–582.
13. Ma YJ, Chen Y, Li L, Cai Z, Wei Z, Jerban S, Jang H, Chang EY, Du J. Trabecular bone imaging using a 3D adiabatic inversion recovery prepared ultrashort TE Cones sequence at 3T. Magn. Reson. Med. 2019; doi: 10.1002/mrm.28027.
14. Horch RA, Gochberg DF, Nyman JS, Does MD. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn. Reson. Med. 2012;68:1774–1784.
15. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013;26:489–506.
Figures