0603
dSAGE enables distortion-free diffusion, spin and gradient echo imaging in 1 minute1State Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, China, 2Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts general hospital, Charlestown, MA, United States, 3Siemens Medical Solutions, Boston, MA, United States, 4Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
We propose dSAGE, a new EPI sequence for diffusion, Spin- and Gradient-echo imaging. We exploit unused sequence time during the b=0 acquisition in a diffusion experiment to collect additional T2*- and T2'-weighted contrasts with high in-plane resolution for free. We use a multi-shot acquisition with high in-plane acceleration to achieve 1x1 mm2 resolution, and alternate the phase-encoding polarities across the shots to eliminate geometric distortion using the navigator-free Blip Up-Down Acquisition(BUDA) technique(1). We demonstrate the ability of BUDA-dSAGE to provide whole-brain, distortion-free, high-SNR images with T2*-, T2'-, T2-weighted contrasts and 3-direction dMRI and apparent diffusion coefficient (ADC) maps in 1-minute.
Introduction
EPI can provide whole-brain coverage rapidly, but does not lend itself to high in-plane resolution imaging due to severe geometric distortion, voxel pile-ups and T2*- or T2-related blurring. This has impaired its utility in high-resolution structural imaging and limited its application to functional or diffusion (dMRI) at lower in-plane resolutions of 1.5-2 mm. Sensitivity encoding allows for up to Rinplane=4 acceleration to mitigate, but not eliminate these artifacts. Recent developments in navigator-free multi-shot EPI reconstruction has allowed for Rinplane8 (2,3), but this requires several shots of data for adequate image quality and fails to eliminate distortion.An alternative strategy is to collect multiple shots with alternating phase-encoding polarities, and reconstruct these jointly using field map information in the forward model as in Hybrid-SENSE (4). BUDA incorporates Hankel structured low-rank regularization into Hybrid-SENSE to eliminate the need for phase navigation (1), and can provide distortion-free dMRI at high in-plane acceleration with minimum T2-blurring.
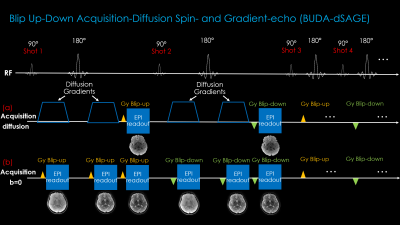
With dSAGE, we propose to utilize the empty sequence time during the b=0 image acquisition. We add two additional EPI readouts to exploit the dead time created by the absence of diffusion gradients. This provides a T2*- and a T2’-weighted contrast image without additional scan time. In essence, we replace the simple b=0 acquisition with a spin- and gradient-echo (SAGE) sequence (5). Combining this with 4-shot BUDA encoding at high Rinplane allows us to create a distortion-free, 1-minute clinical scan at high in-plane resolution (1x1x4 mm3) with gradient-, spin-, and mixed-echo images as well as 3-direction dMRI.
Methods
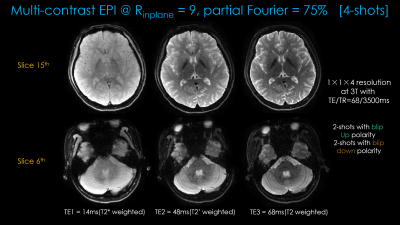
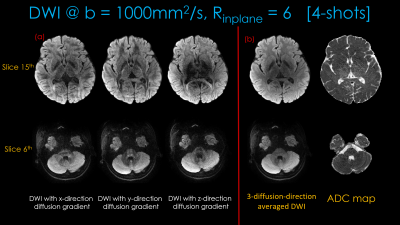
Fig1 shows the proposed prototype sequence diagram where two additional readouts, with gradient and mixed gradient- spin echo contrasts, are added in the unused sequence time during the b=0 acquisition. Four shots of EPI are acquired for each diffusion weighted or b=0 image, where the alternating shots are sampled with opposite phase-encoding polarities. This way, two blip-up and two -down shots are collected for BUDA reconstruction.Acquisition: Data were collected on a 3T Siemens Prisma (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using 32-channel reception with FOV = 220×220×128mm. 1×1×4mm3 resolution b=1000s/mm2 diffusion data were acquired at Rinplane=6 with 4-shots using TE/TR=68/3500ms. The diffusion gradients were applied along the x-, y- and z-directions. b=0 acquisition was made at Rinplane=9 and partial Fourier=6/8, which provided two additional echoes. 4-shots were acquired at the TEs = [14 48 68]ms.
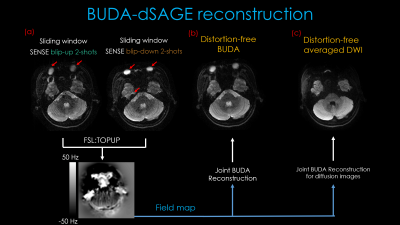
Reconstruction: For spin-echo contrast where shot-to-shot phase variations are minimal due to the refocusing pulse, we first sum the k-space data for the two blip-up and the two blip-down shots and perform SENSE reconstruction to generate interim blip-up and blip-down images. We then estimate a field map using topup (6) and incorporate this in the joint BUDA reconstruction of the 4-shots in the other contrasts:
$$min_x \sum_{t=1}^{N_{s}}{ \left \| {F_{t}W_{t}Cx_{x}-d_{t}} \right\|}_2^2 + \lambda \left \| {H(x)} \right\|_*$$
Where Ft is the undersampled Fourier operator in shot t, Wt is distortion operator in shot t, C is sensitivity map from ESPIRIT [2], and dt is the k-space data of each shot. ||H(x)||* is Hankel structured low-rank constraints. The 3-direction diffusion averaged DWI and ADC maps were calculated after the joint BUDA reconstruction.
Results
Figure 3 shows distortion-free high-resolution multi-contrast images including T2*-, T2- and T2-weighted images.Figure 4(a) shows distortion-free dMRI obtained with diffusion gradients applied along the x-, y- and z-directions. The averaged dwi images and ADC maps are shown in Figure4(b).
The total acquisition time for all contrasts was 56s, plus a 2s calibration scan for coil sensitivities.
Discussion & Conclusion
We demonstrated distortion-free multi-contrast clinical images and dMRI at high in-plane resolution from a single, 1-minute scan. High geometric fidelity of the images can be appreciated in the lower slices in Figs 3 and 4. As the shot-to-shot variations are higher in dMRI, we limited the acceleration to Rinplane=6 to facilitate high quality reconstruction. Structural images supported yet higher (Rinplane=9) acceleration with 6/8 partial Fourier, which minimized their readout duration and allowed them to fit in the unused sequence time. As in single-shot SAGE imaging, we anticipate that these echoes will lend themselves to T2 and T2* parameter mapping, further improving the utility of this sequence.Acknowledgements
This work is supported in part by the National Natural Science Foundation of China (No: U1809204, 61525106, 61427807, 61701436), by the National Key Technology Research and Development Program of China (No: 2017YFE0104000, 2016YFC1300302), and by Shenzhen Innovation Funding (No: JCYJ20170818164343304, JCYJ20170816172431715), and by:NIBIB Award Number: P41 EB015896, R01 EB017337, R01 EB019437, R01 EB020613 and U01 EB025162;NIMH, Award Number: R01 MH116173 and R24 MH106096;Shared instrumentation grants S10-RR023401 and S10- RR023043; andNVIDIA GPU grants. Zijing Zhang is supportedby the China Scholarship Council for 2-years study at Massachusetts General Hospital.References
[1] Bilgic B, Liao C, Manhard MK, et. al, Robust high-quality multi-shot EPI with low-rank prior and machine learning, 2019.
[2] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014;71:990–1001.
[3] Schmiedeskamp H, Straka M, Newbould R D, Zaharchuuk G, et. al, Combined Spin- and Gradient-Echo Perfusion-Weighted Imaging. Magn. Reson. Med. 2012.
Figures