0585
High resolution diffusion and perfusion MRI of normal, preeclamptic and growth-restricted mice models reveal clear fetoplacental differences1Weizmann Institute of Science, Rehovot, Israel
Synopsis
DWI can evaluate pregnancy-related dysfunctions, yet EPI’s sensitivity to motions and air/water/fat heterogeneities complicate these studies in preclinical settings. We have developed DWI methodologies based on SPatiotemporal ENcoding (SPEN) for overcoming these obstacles, delivering single-shot images at ≈100µm in-plane resolutions. These methods were used to monitor fetoplacental differences between naïve and knockout mice strains mimicking preeclampsia and IUGR. High definition ADC/DTI maps could resolve the placental layers (maternal, fetal, trophoblastic), umbilical cords, and various brain compartments in the developing fetuses. Daily monitoring also showed differences in the development of placental and fetal (e.g. brain) structures among normal and disease models.
INTRODUCTION
Understanding the fetoplacental unit, its functional dynamics and development, requires characterizing the transport of fluids within and between maternal, placental and fetal compartments. Improvements in this understanding rest heavily on animal models, which provide a flexibility unavailable in human investigations. Diffusion-weighted imaging (DWI) methods providing ADC/DTI maps without the need for optical access or exogenous agents, could be crucial for this. DWI typically relies on echo-planar-imaging (EPI) single-shot sequences; however, due to tissue/fat/air interfaces and motion, EPI and related techniques have problems to deliver this information in the challenging conditions placed by the abdomens of pregnant, living rodents. We have recently shown that spatiotemporal encoding (SPEN) techniques1 can cope with this kind of limitations, and lead to DWI maps of fetoplacental units in mice with excellent 3D spatial resolution.2 Indeed, although initial single-shot SPEN studies on pregnant animals were resistant to a certain measure of field heterogeneities and of motions, they suffered from limited sensitivity that in term limited the spatial resolution that they could achieve; those measurements were thus mostly restricted to pregnant rats.3 We have since, however, developed optimized interleaved approaches that, by allowing one to combine multiple complementary data, can deliver diffusivity maps in living rodents with ~80µm isotropic resolution.4 This in turn allowed us to port diffusivity measurements from rats to mice, thereby opening new opportunities in terms of our potential to study animal models. The power of these new methods is here used to compare the development between naïve and vascularly-altered mice. The latter were assessed for two knock-out mice models: eNOS (endothelial nitric oxide synthase) deficient (-/-) animals, which are associated with intrauterine growth-restriction (IUGR) symptoms; and IL10 knockout mice, exhibiting hypertension and proteinuria during pregnancy, and serving as a model for preeclampsia (PE). Experiments were extended to a chemically-induced model of IUGR PE, arising from the injection of N(gamma)-nitro-L-arginine methyl ester (l-NAME, an NOS inhibitor) on naïve (black) mice. Strong modulations could be observed when comparing these IUGR and PE models vs naïve animals in the placental ADCs mapped at 7T – particularly in the distributions adopted by the ADC values throughout the maternal and fetal layers of this organ. Related effects could be noted when performing kinetic measurements utilizing gadolimium-based T1 contrast agents, which also hinted at differences in perfusion within these placental structures. High resolution 3D ADC and DTI maps were also obtained with new SPEN pulse sequences –operating at 15.2T and including self-navigators– on in vivo pregnant mice E14.5; although no significant differences could be observed between the development of the disease and naïve animals, clear fiber direction maps of the fetal brain were obtained.METHODS
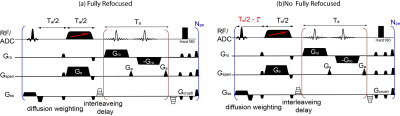
Both multi-slice and 3D phase-encoded versions of SPEN were used in this study (Fig. 1). For the 3D DTI SPEN sequence a 2D navigator echo applied at the conclusion of the scan was used to correct unwanted phase errors induced by motion during the diffusion gradient, a provision demanded due to the multiscan with phase encoding. Daily pregnancy development was monitored on control and on the pregnancy-impaired mice. Diffusion weighted MRI ADC mapping and DCE-based perfusion experiments were performed from gestation day E10.5 onwards on a 7T Agilent scanner; 3D-DTI high resolution experiments were performed on a 15.2T Bruker scanner for achieving a higher SNR.RESULTS & DISCUSSION:
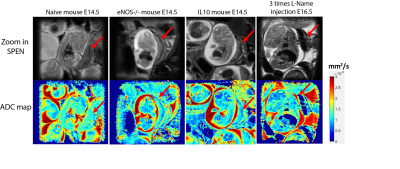
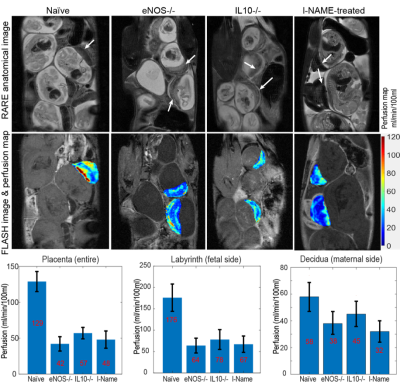
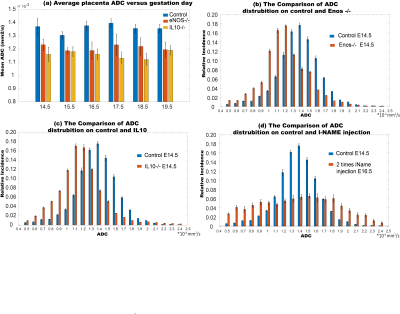
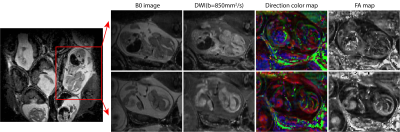
Figure 2 shows SPEN-based diffusion measurements performed for different mice model, including b0 (anatomical) images of the animal models that were targeted, together with the corresponding SPEN-derived ADC maps. These measurements zoomed-in into single fetoplacental regions of interest, of which many could be identified in each dam. It is interesting to compare the placental features arising from these diffusion measurements, against the perfusion experiments that based on the contrast imparted by Gd-DTPA are observed in the DCE MRI results shown in figure 3. In both DWI and DCE-MRI we can notice three main placental layers: a labyrinth region proximate to the fetus that is irrigated by the larger maternal spiral arteries, and a decidual region that is separated from the former by a pearled, thin, low-diffusivity giant throphoblast cell structure. Notice that the labyrinth shows both the fastest levels of perfusion as well as the fastest ADCs. As Figure3 and Figure 4 also shows, the diffusion ADCs and perfusion rates are very different for the naïve control animals, than for the disease model ones; in all cases, fluid exchanges in the latter are compromised. These differences remain more or less constant throughout the pregnancy. Also, the effects of health and diseased pregnancies were investigated for the fetuses themselves; Figure 5, for instance, shows the kind of 3D DTI data that for in vivo pregnant mice could be recorded on fetal brains at 15.2T. These results will also be discussed.CONCLUSION
The valuable insight could arise from ADC and DTI characterizations of in vivo pregnancy in health and disease. Systematic developmental and physiological studies of dam and fetus changes become feasible, and correlations with symptoms and disease phenotypes emerge. SPEN could prove a valuable aid in both preclinical and human studies of this type; preclinical acquisition and processing software packages for collecting this kind of data is available in https://www.weizmann.ac.il/chemphys/Frydman_group/software.Acknowledgements
We are grateful to Dr Zhiyong Zhang & Dr. Tangi Roussel (Weizmann/CEA) for their help with the programming. The authors also acknowledge the support from the Israel Science Foundation (grants 2508/17 and 965/18), the Kimmel Institute for Magnetic Resonance (Weizmann Institute) and the generosity of the Perlman Family Foundation.References
1. Tal A, Frydman L, Spatial encoding and the single-scan acquisition of high definition MR images in inhomogeneous fields. Journal of Magnetic Resonance. 2006; 182:179–194.
2. Q. Bao, E. Solomon, G. Liberman and L. Frydman, ”High definition diffusion MRI: Principles and applications to visualizing pregnant mice development”; NMR Biomed., in press (2019).
3. Solomon, E., et al., Major mouse placental compartments revealed by diffusion-weighted MRI, contrast-enhanced MRI, and fluorescence imaging. Proc Natl Acad Sci U S A, 2014. 111(28): p. 10353-8. 4. M. Yon, Q. Bao, R. Henriques, N. Shemesh and L. Frydman, “High-resolution 3D in vivo mouse brain Diffusion Tensor Imaging at ultrahigh fields: A comparison of methods”; submitted for publication (2019).
5. Schmidt, R. and L. Frydman, New spatiotemporal approaches for fully refocused, multislice ultrafast 2D MRI. Magn Reson Med, 2014. 71(2): p. 711-22.
6. Schmidt, R., A. Seginer, and L. Frydman, Interleaved multishot imaging by spatiotemporal encoding: A fast, self-referenced method for high-definition diffusion and functional MRI. Magn Reson Med, 2016. 75(5): p. 1935-48.
7. Bonel, H.M., et al., Diffusion-weighted MR Imaging of the Placenta in Fetuses with Placental Insufficiency. Radiology, 2010. 257(3): p. 810-819.
Figures