0583
Placenta Accreta Spectrum (PAS) Disorders Investigated with Multi-Compartment Placental MRI1Institute for Women's Health, University College London (UCL), London, United Kingdom, 2School of Biomedical Engineering and Imaging Sciences (BMEIS), Kings College London, London, United Kingdom, 3University College London Hospital, London, United Kingdom, 4Department of Medical Physics and Biomedical Engineering, University College London Hospital (UCLH), London, United Kingdom, 5Great Ormond Street Hospital for Children, London, United Kingdom, 6University Hospitals, KU Leuven, Leuven, Belgium, 7Centre for Medical Imaging (CMI), University College London (UCL), London, United Kingdom, 8Department of Medical Physics and Biomedical Engineering, University College London (UCL), London, United Kingdom
Synopsis
Uterine scarring from caesarean section (CS) can lead to subsequent abnormally adherent or invasive placenta. Failure to recognise Placenta Accreta Spectrum disorders prior to delivery can potentially cause catastrophic bleeding and death. Complex surgical interventions may be required to remove placental invasion of the uterine myometrium and nearby organs. Antenatal detection and correct PAS grading are important to plan delivery. Current ultrasound and MRI imaging are limited to subjective assessment of vascular invasion. We propose a multi-compartment model1 that can quantify vascularity and proportion of abnormal placentation across the previous CS scar for objective diagnosis and to assist surgical planning.
Introduction
Placenta Accreta Spectrum (PAS) Disorders involve abnormal placental adherence and invasion into the myometrium or potentially through uterine serosa and into surrounding organs such as the bladder, bowel and pelvic side wall2,3. This pathology is more likely when mothers have one or more previous caesarean sections and has a prevalence of 1.7 per 10,000 pregnancies2.The placenta is separated from the myometrium by the decidua4. Injury to the endometrium, through uterine surgery such as caesarean section, can result in a decidual deficit in subsequent pregnancies causing abnormal placentation5,6. Failure to recognise and appropriately manage PAS disorders at delivery can lead to massive post-partum haemorrhage with a mortality rate of 2.6-7% as the placenta fails to detach from the uterine wall and surrounding tissues7. It is important to correctly diagnose and manage this disorder, with accurate assessment of extent of invasion for better surgical planning. This is being performed by subjective interpretation of typical sonographic markers, with MRI only used as an adjunct. Some structural MRI signs include dark T2 intraplacental bands, placental heterogenous signal intensity, uterine bulging, focal interruption of the myometrium and tenting of the bladder6,8. These morphologic markers are highly subjective, even when advanced MRI methods such as DWI, and IVIM have been employed9.The limitation of these methods includes low resolution and difficulty assessing vascular invasion9-11. We propose a novel MRI multi-compartmental model to investigate vascularity and invasion in PAS disorders. We used a combined T2 relaxometry (T2R) and intravoxel incoherent motion (IVIM) signal model, DECIDE1, separating signals from fetal and maternal blood pools over a region of interest of previous scar tissue and suspected abnormal placentation, the placenta-utero-bladder interface1,12. By exploring how parameters from this multi-compartmental model differ between PAS disorders and controls, we aim to identify differences in vascularity and perfusion to an objective quantification of abnormal placentation and tissue invasion.Methods
Five women with suspected PAS disorders diagnosed on mid-trimester ultrasound, using a standardised protocol13, consented to fetal MRI in the third trimester (29+4 to 38+2 weeks gestation). Images were compared to five control patients without PAS (28+2-30+1 weeks gestation). Imaging was performed on 1.5T Siemens Symphony, taking 41 measurements grouped across 7 b-values (0, 50, 100, 150, 200, 400, 600 s/mm²) acquired at TE=96ms and 10 echo times (81, 90, 96, 120, 150, 180, 210, 270, 300ms) with b-value of 0, 50 and 200 s/mm2. The imaging plane was selected to cover the depth of placenta, from chorionic to basal plate.The whole placental volume was imaged. A 3D non-rigid registration routine was used to align images14, and manual segmentation of regions of placenta, myometrium, and placenta-utero-bladder interface was performed. The retroplacental myometrium was segmented in controls. Paired t-test was performed to compare mean differences between groups. Statistical significance was set at 5%.Results
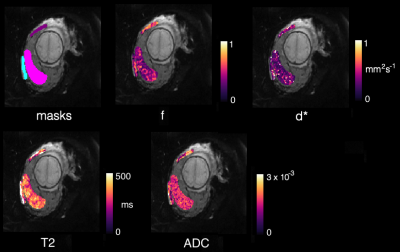
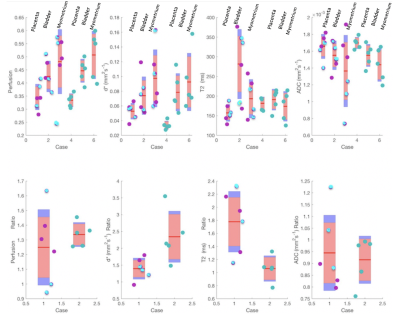
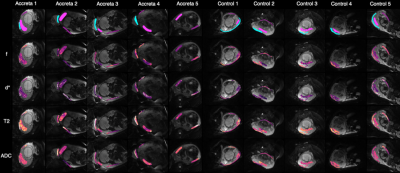
All patients with suspected PAS disorders had low-lying placentation. Two had histopathological confirmation, and one, a clinical diagnosis on examination of the uterus at delivery. Two had low-lying placentation with no evidence of adherence or invasion.Figure 1 shows raw masks and parametric maps for one PAS case.Figure 2 shows the perfusion fraction (f), pseudo-diffusivity (d*), T2, and ADC plotted against each PAS disorder, low-lying placenta, and controls. The T2 appears to be elevated, and the d* reduced in PAS disorders in comparison to controls. The placenta-utero-bladder interface/placenta ratio shows the proportion of placental tissue signal in the interface. This is significant in T2(ms) ratio (p=0.0109, (5%CI = 0.209, 1.2230)), and d*(mm2s-1) ratio (p=0.0207, (5%CI=-1.715,-0.182)).Figure 3 shows parametric maps for all accreta and control cases. The d* values appear visibly reduced, whilst T2, appears increased in patients with PAS disorders in comparison to controls.Discussion
Using our MRI multi-compartmental model, we have found that women with PAS disorders show significantly reduced pseudo-diffusivity (d*) at the utero-placenta-bladder interface compared to controls. T2 appears significantly elevated in patients with PAS disorders in comparison to controls. There were no significant differences in perfusion fraction (f), and ADC parameters between the two groups. Scarring from previous caesarean sections can lead to decidual deficiency causing abnormal vascularisation, and hypoxia which may lead to defective decidualisation and abnormal trophoblastic invasion6.The volume of the intervascular space which is filled with maternal blood may therefore decline, leading to a decrease in pseudo-diffusivity (d*). Moreover, T2 appears increased in PAS disorders in comparison to controls. These results may be related to thinning of the decidual-myometrial-bladder interface and increased vascular motion in these tissues. This may be indicative of the vascular tendency of this area during surgery which may aid surgical planning.Conclusion
This study investigated the use of parametric MRI to quantify the degree of invasive placentation in PAS disorders. Although we do not find a clear pattern of differentiation between PAS disorders, low-lying and normal placentation, in principle quantitative MRI is sensitive to both the vascular changes and proportion of invasion whilst avoiding the bias of subjectivity in relation to currently recommended structural MRI signs. Further work with a larger sample size may allow us to better understand the physiological changes in this complex pathology and inform surgical planning.Acknowledgements
This work is part of the Guided Instrumentation of Fetal Therapy and Surgery (GIFT-Surg) project, funded by the Wellcome Trust [203148/Z/16/Z; 203145Z/16/Z; WT101957] and Engineering and Physical Sciences Research Council (EPSRC) [NS/A000049/1; NS/A000050/1; NS/A000027/1; EP/L016478/1]. Wellcome/EPSRC centre for Interventional and Surgical Sciences (WEISS)References
1. Melbourne, A., et al., Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med, 2019. 81(1): p. 350-361.
2. Jauniaux, E., et al., FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynaecol Obstet, 2018. 140(3): p. 274-280.
3. Jauniaux, E., et al., FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet, 2019. 146(1): p. 20-24.
4. Cuthbert, F., M. Teixidor Vinas, and E. Whitby, The MRI features of placental adhesion disorder-a pictorial review. Br J Radiol, 2016. 89(1065): p. 20160284.
5. Tantbirojn, P., C.P. Crum, and M.M. Parast, Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta, 2008. 29(7): p. 639-45.
6. Shetty, M.K. and D.K. Dryden, Morbidly Adherent Placenta: Ultrasound Assessment and Supplemental Role of Magnetic Resonance Imaging. Semin Ultrasound CT MR, 2015. 36(4): p. 324-31.
7. Goergen, S.K., et al., Interobserver agreement and diagnostic performance of individual MRI criteria for diagnosis of placental adhesion disorders. Clin Radiol, 2018. 73(10): p. 908 e1-908 e9.
8. D'Antonio, F., et al., Prenatal identification of invasive placentation using magnetic resonance imaging: systematic review and meta-analysis. Ultrasound Obstet Gynecol, 2014. 44(1): p. 8-16.
9. Millischer, A.E., et al., Dynamic contrast enhanced MRI of the placenta: A tool for prenatal diagnosis of placenta accreta? Placenta, 2017. 53: p. 40-47.
10. Kilcoyne, A., et al., MRI of Placenta Accreta, Placenta Increta, and Placenta Percreta: Pearls and Pitfalls. AJR Am J Roentgenol, 2017. 208(1): p. 214-221.
11. Morita, S., et al., Feasibility of diffusion-weighted MRI for defining placental invasion. J Magn Reson Imaging, 2009. 30(3): p. 666-71.
12. Jerome, N.P., d’Arcy, J A., Feiweier T., Koh, D-M. , Leach M O., Collins D J., Orton M R., Extended T2-IVIM model for correction of TE dependance of pseudo-diffusion volume fraction in clinical diffusoon-weighted magnetic resonance imaging. Phys Med Biol, 2016. 61: p. N667-N680.
13. Collins, S.L., et al., Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol, 2019. 220(6): p. 511-526.
14. Flouri, D., Owen, D., Aughwane, R., Mufti, N., Sokolska, M., Atkinson, D., Kendall, G., Bainbridge, A. , Vercauteren, T., David, A.L., Ourselin, S., Melbourne, A., Improved Placental Parameter Estimation Using Data-Driven Bayesian Modelling. Med Image Comput Assist Interv, 2019(11766): p. 609-616.
Figures