0582
Placental perfusion in Fetuses with congenital heart disease and normal pregnancy assessed with intravoxel incoherent motion imaging1Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 2MR Collaboration, Healthcare Siemens Ltd., Beijing, China, Beijing, China, 3Healthcare GmbH, Erlangen, Germany, Erlangen, Germany
Synopsis
To determine whether placental perfusion alterations are evident in utero in fetuses with congenital heart disease (CHD), we quantitatively investigated perfusion in 28 fetuses with CHD and 39 healthy gestational age–matched controls using intravoxel incoherent motion imaging (IVIM). We found that the f values were significantly higher in the CHD group compared with the normal pregnancy group (37.8% vs. 30.2%, p<0.0001), and there was no significant difference in the D value and D* value between the two groups. The increased placental perfusion in fetuses with CHD might represent an attempt to compensate for a perfusion deficit in fetal circulation.
Introduction
The placenta is a vital organ that provides oxygen and nutrients, discharges wastes, which are critical for normal fetal neurodevelopment[1]. While placental insufficiency is known to often result in fetal growth restriction (FGR) or preeclampsia, the relationship between placental function and congenital heart disease (CHD) in fetuses is largely unknown[2,3]. Intravoxel incoherent motion imaging (IVIM) applies a bi-exponential model to evaluate both capillary perfusion and tissue diffusion[4]. Therefore, in this study, we aimed to explore placental perfusion in fetuses with CHD and gestational age (GA)–matched healthy fetuses using IVIM.Methods
This prospective study was approved by our Institutional Review Board, and all patients provided written informed consent. From January 2019 through October 2019, fetuses with CHD (screened by US) and GA-matched healthy controls that had undergone placenta examinations were enrolled. The MRI examinations were performed on a 1.5T MRI system (MAGNETOM Amira, Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China) with a 13-channel body coil. A single-shot echo-planar imaging sequence with 8 b-values (0, 50, 100, 150, 200, 250, 500, and 800s/mm2) using a free-breathing technique was applied to acquire IVIM data in the transverse plane of the placenta. The acquisition parameters were: TR = 4700ms, TE = 93ms, FOV = 300 × 300 mm2, slice thickness = 5mm, matrix = 168 × 168, iPAT factor = 2. Diffusion gradients were encoded in three directions. The acquisition time was 2:36 min.IVIM-derived perfusion fraction (f), pseudo-diffusion coefficient (D*), and standard diffusion coefficient (D) parametric maps were generated offline using prototypic postprocessing software (MR Body Diffusion Toolbox, Siemens Healthcare, Erlangen, Germany). Regions of interest (ROIs) were drawn encircling the entire placenta bound by the decidual and chorionic plates, but not including the endometrium and large vessels. All measurements (D, D*, and f) were performed by two radiologists. All data were expressed as means ± standard deviation (SD). Kappa statistics were used to evaluate measurement consistency between observers. The f, D, and D* values of the CHD group, and GA-matched healthy controls were compared using two independent samples t-tests. Statistical analyses were performed using SPSS (V17, IBM Corp., Armonk/NY, USA). A P < 0.05 was considered statistically significant.
Results
A total of 71 pregnant women were enrolled, 32 with pregnancies complicated by CHD of fetuses, and 39 GA-matched healthy controls. Of these, three cases from the CHD group were excluded because (a) the fetuses were subsequently diagnosed with significant extra-cardiac anomalies, two with chromosomal anomalies, and one with nodular sclerosis) and (b) 1 case had incomplete MRI data. In the remaining 67 cases (28 fetuses with CHD, 39 fetus without CHD), all the MRI examinations were performed in the second trimester of pregnancy. The median GA was 26 weeks (range 23–27 weeks), and the median age of pregnant women was 25 years (range 23–41years). The CHD structural lesions included double-outlet right ventricle, complete transposition of the great arteries, hypoplastic left heart syndrome, aortic stenosis, coarctation of aortic arch, single cardiac ventricle, double aortic arches, interventricular septal defect, atrial septal defect, and tetralogy of fallot.The kappa coefficients (Inter-observer agreement) regarding the f, D and D* measurements were 1.0 (95% CI: 1.0-1.0), 0.9 (95% CI: 0.8-1.0), and 1.0 (95% CI: 1.0-1.0), respectively. There were no significant differences and excellent inter-observer agreement among the three parameters (all 95% CI overlapped).
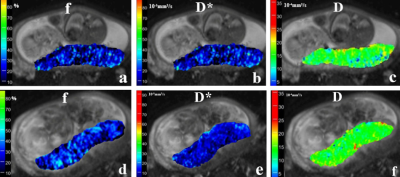
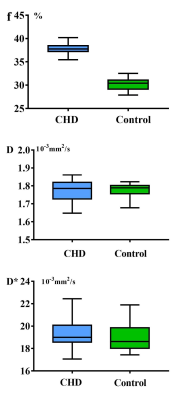
The f, D and D* parametric maps were shown in Figure 1. In the CHD group, the f values were 37.8 ± 3.5%, D values were 1.77 ± 0.07 (×10-3 mm2/s), and D* values were 19.38±1.41 (× 10-3 mm2/s), respectively. In the control group, the f values were 30.2±2.1%, D values were 1.78±0.05 (× 10-3 mm2/s) and D* values were 18.96±1.56 (× 10-3 mm2/s), respectively. The results showed that no significant difference in the D value and D* values between the CHD and control groups (p=0.71, p=0.14, respectively) were present. However, the f values were significantly higher in the CHD group compared with those of the healthy controls (37.8% v.s. 30.2%, p<0.0001) (Figure 2).
Discussion and Conclusion
Our findings demonstrated that placental perfusion in fetus with CHD was significantly different compared with controls. Similar to the human brain, heart, and kidney, the placenta might have an autoregulatory mechanism to maintain optimal perfusion[3]. The increased placental perfusion in fetuses with CHD could represent an attempt to compensate for fetal circulation and perfusion deficits, which could eventually fail in the third trimester of pregnancy. In summary, our results showed that IVIM provided new options to evaluate placental microcirculation accurately. The increased perfusion fraction could distinguish pregnancies with fetal CHD from normal pregnancies in the second trimester of pregnancy.Acknowledgements
We are grateful to all the participants and their families who consented to participate in this study. We thank the radiography staff at the Shandong Medical Imaging Research Institute, Shandong University, for their support in imaging data collection and processing.References
1. Maslen, C. L. Recent Advances in Placenta–Heart Interactions. Front. Physiol. 9, 735 (2018).
2. Courtney, J. A., Cnota, J. F. & Jones, H. N. The Role of Abnormal Placentation in Congenital Heart Disease; Cause, Correlate, or Consequence? Front. Physiol. 9, 1045 (2018).
3. Zun, Z., Zaharchuk, G., Andescavage, N. N., Donofrio, M. T. & Limperopoulos, C. Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Sci. Rep. 7, 16126 (2017).
4. Federau, C., O’Brien, K., Meuli, R., Hagmann, P. & Maeder, P. Measuring brain perfusion with intravoxel incoherent motion (IVIM): Initial clinical experience: Brain IVIM: Initial Clinical Experience. J. Magn. Reson. Imaging 39, 624–632 (2014).
Figures