0581
Placental MRI: Effect of maternal position, breath hold and oxygen state on placental T2* measurements1Fetal-Neonatal Neuroimaging & Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Computer Science and Artificial Intelligence Laboratory (CSAIL), Massachusetts Institute of Technology, Cambridge, MA, United States, 7Pathology, Massachusetts General Hospital, Boston, MA, United States, 8Maternal-Fetal Medicine, Massachusetts General Hospital, Boston, MA, United States
Synopsis
T2* relaxometry has been proposed as a semi-quantitative measure for placental oxygen transport. However, to use T2* as a diagnostic tool, it is necessary to define the normal range of results and factors that influence those results. In this study we investigated the effect of maternal position, breath-holds and oxygen state on placental T2*. We observed lower T2* with breath-hold protocol compared to no breath-hold protocol in left lateral position. Additionally, lower T2* was measured in supine position during normoxic episode compared to left lateral position with no breath-hold protocol. Further studies are needed to understand these factors better.
Purpose:
The ultimate goal of placental MRI is to improve individual patient care by accurately characterizing individual placental function. T2* relaxometry has been proposed as a semi-quantitative measure of the spatiotemporal patterns of placental oxygen delivery and transport between the mother and the fetus, which may enable us to better understand the function of the placenta and to detect placental insufficiency.1-5 To use T2* measurements as a diagnostic tool, the initial phase requires defining the normal range of T2* values and factors that influence placental T2* maps. In this study, we investigated the effect of maternal position, breath holds and oxygen state on placental T2* measurements.Methods:
Subjects: 8 subjects, 7 with normal singleton pregnancies and 1with high-risk pregnancy (gestational age: 25-38 weeks) were included. Acquisitions: Scans were performed on a 3T Skyra scanner (Siemens Healthcare, Erlangen, Germany). 4 of the subjects were asked to switch position (supine to left lateral or vice versa) during the scan and the same MRI protocol was applied in both positions to compare the effect of maternal positioning on T2* measurements. For the remainder (3 normal pregnancies and 1 high risk pregnancy), data were collected only in the left lateral position. Time course, no breath-hold, T2* were obtained using a multi-echo (TEs: 18, 47.8, 77.5ms) GRE EPI sequence (TR: 4.2s, in-plane resolution of 3.1mm×3.1mm, a slice thickness of 3mm, 70 slices, GRAPPA 2, SMS 2) during an initial normoxic episode (room air, 21% O2, 5min), followed by a hyperoxic episode (100% FiO2, 3min). Single time point breath-hold T2* measurements were obtained just before the time course above using a multi-echo (TEs: 18, 47.8, 77.5ms) GRE MRI sequence (TR: 384ms, in-plane resolution of 3mm×3mm, a slice thickness of 3mm with 7.5mm slice gap, 12 slices, GRAPPA 2, total acquisition time: 2.3min with 6 breath-holds 13s each). For 4 subjects participating single position protocol, another breath-hold T2* measurement was collected at the end of hyperoxic episode. Processing and Analysis: We corrected signal non-uniformity and motion in the dynamic multi-echo GRE EPI series using our previously reported computational pipeline.6 Voxel-wise T2* maps were calculated by fitting a mono exponential decay model to the measured intensities and their corresponding echo times. We employed the Student t-test for group comparisons between T2* values collected during normoxic episode in left lateral and supine positions and also between T2* values collected with and without breath-hold acquisitions during normoxic and hyperoxic episodes.Results and Discussion:
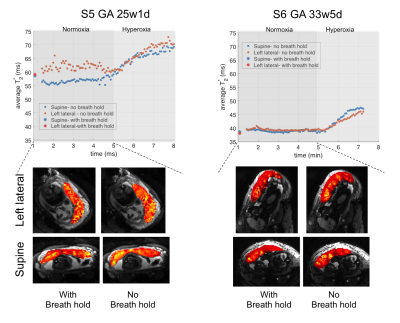
Data from 2 normal subjects participated in the positioning protocol were excluded due to the extreme fetal motion. The other 6 data sets were successfully analyzed. Figure 1 demonstrates the results of 4 subjects participating the single position protocol. We observed significantly lower placental T2* values with breath-hold (p=0.038). Higher contrast between periphery and center of cotyledon structures was observed in breath-hold protocol compared to no breath-hold protocol as shown with single slice T2* maps. Note that for subject 2, at the end of hyperoxic episode we observed a non-labor uterine contraction not reported by the mother resulting in a decrease in T2* estimate (i.e. average T2* = 30.5ms) lower than the baseline measurement (i.e. average T2* = 31.5ms). For subject 4, high risk pregnancy, we observed lower T2* values both in breath-hold and no breath-hold protocols compared to the control subjects at the similar gestation age (i.e. 32weeks). Figure 2 shows the average T2* plots for two normal subjects in two different maternal positions. For both subjects we observed lower T2* estimates in supine position compared to the left lateral position during normoxic episode when no breath hold protocol was used. Increase in T2* estimates during hyperoxic episode was higher in supine position (i.e. For subject 5 ΔT2*supine=12.7ms, ΔT2*left = 8.2ms; For subject 6 ΔT2*supine= 8.2 ms, ΔT2*left = 6.6ms). In the left lateral position T2* estimates were lower with the breath-hold protocol, but in the supine position we observed very similar values with both protocols such that for subject 5 (with positioning order as supine first, and left lateral second) T2* with breath-hold was higher than no breath-hold and for subject 6 (with positioning order as left lateral first, and supine second) estimates were similar. This can be also related with the duration spent in each position and the order of the positioning. Further studies and more subjects are needed to better understand these factors.Conclusion:
We show that placental T2* measures vary with maternal position, breath-hold and oxygen state. In addition, occult contractions contribute to T2* variability. In order to develop T2* mapping as a diagnostic tool and decrease variance in placental T2* measures, the impact of these factors needs to be well characterized in both healthy and pathologic placentas.Acknowledgements
NIH U01 HD087211, NIH R01 EB017337, NIH P41 EB015902.References
1. Hutter, Jana, et al. Multi-modal functional MRI to explore placental function over gestation. Magnetic resonance in medicine 81.2 (2019): 1191-1204.
2. Sinding, Marianne, et al. Placental baseline conditions modulate the hyperoxic BOLD-MRI response. Placenta 61 (2018): 17-23.
3. Ingram E, Morris D, Naish J, et al. MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology. 2017;285:953–960.
4. Huen I, Morris DM, Wright C, et al. R1 and R2* changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med. 2013;70:1427–1433.
5. Poulsen SS, Sinding M, Hansen DN, et al. Placental T2* estimated by magnetic resonance imaging and fetal weight estimated by ultrasound in the prediction of birthweight differences in dichorionic twin pairs. Placenta. 2019;78:18–22.
6. Abaci-Turk E et al. Spatiotemporal alignment of in utero BOLD-MRI series. Journal of Magnetic Resonance Imaging. 2017 Aug; 46(2):403-12.
Figures