0577
Quantitative Perfusion Measurements of the Human Placenta with FAIR and pCASL Arterial Spin Labeling at 3T: Initial Feasibility1Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Obstetrics and Gynecology, UT Southwestern Medical Center, Dallas, TX, United States, 3Population and Data Sciences, UT Southwestern Medical Center, Dallas, TX, United States, 4Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
Quantitative measurement of placental perfusion is important for the assessment of placental function. We have developed and optimized a non-contrast perfusion MR imaging technique utilizing pseudo-continuous arterial spin labeling (pCASL) to quantitatively measure human placental perfusion at 3T. Placental perfusion was also assessed using flow-sensitive alternating inversion recovery (FAIR). The average placental blood flow (108±47 mL/100g/min) was comparable to published literature values.
Introduction
The placenta plays a critical role in the health of both mother and developing fetus (1,2). Understanding of the development and maturation of the human placenta during pregnancy is sorely lacking. Quantitative measurement of placental perfusion provides insight of placental function. Noninvasive perfusion magnetic resonance (MR) imaging is used in human placental research to minimize risk to the fetus. Arterial spin labeled (ASL) MRI has been extensively studied in the brain, its application to body imaging has been limited due to technical challenges associated with larger anatomical coverage and physiological noise such as respiration. The first placental ASL study was conducted using Flow-sensitive Alternating Inversion Recovery (FAIR) (3). Placental perfusion measured by FAIR contains both the maternal and fetal flow, thus limiting assessment solely of maternal blood supply. FAIR also has relatively low signal-to-noise ratio (SNR). Another ASL technique, pseudo-continuous ASL (pCASL) is capable of selectively labeling the maternal feeding aorta and thus captures the maternal contribution of placental perfusion. Compared to FAIR, pCASL has been shown to have higher SNR. The purpose of this work was to demonstrate the feasibility of non-invasive whole-placenta perfusion imaging using ASL-MRI in utero at 3T. Placental perfusion in the 2nd and 3rd trimesters will be investigated. The study is approved by the local IRB.Methods
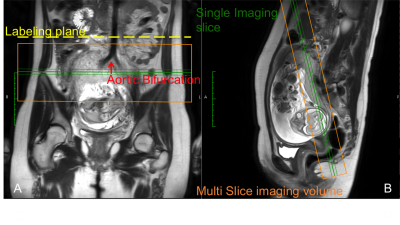
Inclusion criteria: singleton pregnancy, no complications, gestation in the early 2nd (18-22 weeks) or 3rd trimester (28-36 weeks), and a normal ultrasound study. MR scans were performed in supine position on a 3T Ingenia MR scanner (Philips Healthcare, Best, the Netherlands), equipped with a body array coil for signal reception.Three-plane T2-weighted images were acquired before the ASL scan to assist the positioning of the labeling plane and imaging volume. ASL-MRI was performed using both pCASL (n=9) and FAIR (n=8) (4). FAIR was performed with a non-selective labeling in the same imaging volume as pCASL. pCASL labeling plane was positioned perpendicularly to the abdominal aorta, just above the aortic bifurcation (Fig 1).
The imaging parameters for pCASL were: TR/TE = 6 sec/24 msec, resolution =3x3 mm2, slice thickness = 10 mm, four pairs of control/label images for each slice, no. of slices = 5, label duration = 1.8 sec, post-label delay = 1.8 sec, total scan time = ~4 min. FAIR used similar imaging parameters with an inversion time of 1.8 sec for a total scan time of ~4 min. Both pCASL and FAIR included background suppression to minimize temporal fluctuations due to motion. Proton-density images for each slice were acquired with the same imaging parameters, but without any labeling and background suppression for perfusion quantification.
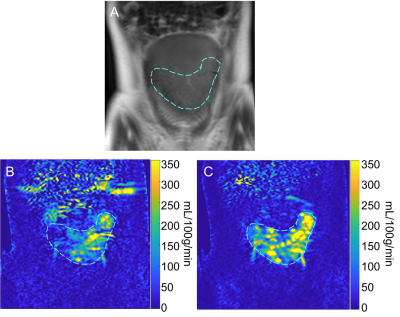
Data analysis was performed offline using Matlab (The Mathworks, Natick, MA). Perfusion weighted images were reconstructed using complex k-space subtraction between the label and the control images followed by homodyne reconstruction. The perfusion signal was measured in a hand-drawn region of interest (ROI) based on T2-weighted control images (Fig 2). Pixel-wise placental blood flow (PBF) was estimated as described previously (5).
Results
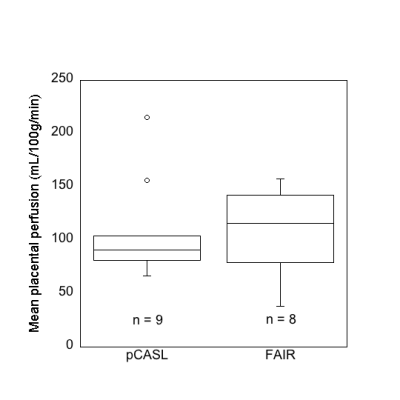
Nine women were imaged, 3 in the 3rd trimester (age: 22±2 y, gestational age: 32±4 weeks) and 6 in the 2nd trimester (age: 26±6 y, gestational age: 20±2 weeks). Mean placental perfusion measured with pCASL was 108±47 mL/100g/min, FAIR measurement was 108±41 mL/100g/min (Fig 3). PBF values of women in 2nd trimester (n=6) and 3rd trimester (n=3) were 83±14 mL/100g/min and 115±35 mL/100g/min.Discussion
In this study, we demonstrated non-invasive placental perfusion quantification in 9 healthy pregnant women using pCASL and FAIR-ASL as a feasibility study in pregnancy. We present the mean placental perfusion in the 2nd and 3rd trimester. Our results for mean placental perfusion across gestational age are comparable to the published studies on ASL-measured placental perfusion in normal pregnancy (3,6-8).Our study was conducted on 3T scanner and multi-slice images were acquired using FAIR and pCASL. Spatial variation of perfusion signal with focal hyper intensities is observed. This heterogeneity in perfusion signals may indicate spatial variation in the vascular structure of the placenta, specifically the placental cotyledons.
In our small series, scans performed within the 2nd trimester were less likely to have severe motion artifacts in perfusion images compared to those in 3rd trimester. This may be a result of the more frequent fetal movement in late pregnancy. Additionally, placental position in the uterus (anterior, posterior, or fundal) critically influences the labeling efficiency and the ability to obtain reliable perfusion weighted image. Overall, women with an anterior placenta generally allow more reliable placental perfusion to be acquired.
From our study, we were able to establish a non-invasive placental perfusion imaging protocol in pregnant women using ASL. We plan to utilize the protocols from this feasibility study in a prospective clinical trial of serial MR placental function in normal and abnormal pregnancy states.
Acknowledgements
No acknowledgement found.References
1. Linduska N, Dekan S, Messerschmidt A, et al. Placental pathologies in fetal MRI with pathohistological correlation. Placenta 2009;30(6):555-559.
2. Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta 2013;34(10):841-845.
3. Gowland PA, Francis ST, Duncan KR, et al. In vivo perfusion measurements in the human placenta using echo planar imaging at 0.5 T. Magnetic Resonance in Medicine 1998;40(3):467-473.
4. Zhang Y, Kapur P, Yuan Q, et al. Tumor Vascularity in Renal Masses: Correlation of Arterial Spin-Labeled and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Assessments. Clinical Genitourinary Cancer 2016;14(1):e25-e36.
5. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015;73(1):102-116.
6. Zun Z, Zaharchuk G, Andescavage NN, Donofrio MT, Limperopoulos C. Non-Invasive Placental Perfusion Imaging in Pregnancies Complicated by Fetal Heart Disease Using Velocity-Selective Arterial Spin Labeled MRI. Scientific Reports 2017;7(1):16126.
7. Shao X, Liu D, Martin T, et al. Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3T. Journal of Magnetic Resonance Imaging 2018;47(6):1667-1676.
8. Zun Z, Limperopoulos C. Placental perfusion imaging using velocity-selective arterial spin labeling. Magnetic Resonance in Medicine 2018;80(3):1036-1047.
Figures