0576
Probing ballistic flow in the placenta using flow-compensated and non-compensated diffusion MRI1Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China, 2Shanghai Key Laboratory of Embryo Original Diseases, Shanghai, China, 3Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 4MR Collaboration, Siemens Healthcare Ltd., Shanghai, China
Synopsis
Intravoxel incoherent motion (IVIM) imaging is frequently used to evaluate microcirculatory flow. With the conventional diffusion MRI, IVIM effects include both pseudo-diffusive microcirculatory flow and bulk (or ballistic) blood flow. We propose a joint use of flow-compensated (FC) and non-FC diffusion gradient waveforms to specifically probe the fraction and velocity of ballistic flow in the placenta. The measured ballistic flow velocity showed a high correlation with umbilical flow based on Doppler ultrasound and a negative correlation with gestational age. These results demonstrated the potential of using FC/NC dMRI to noninvasively measure flow velocity inside the placenta.
Introduction
Intravoxel incoherent motion (IVIM) has shown to be a useful tool in assessing the microcirculatory flow in the brain and body organs, including the placenta 1,2. Conventional IVIM uses monopolar or double-refocused diffusion sensitization gradients, which are non-flow compensated. Thus, the measured IVIM effects include both the pseudo-diffusive microcirculatory flow and the bulk blood flow (or ballistic flow) 3,4. In this study, we investigated a joint use of flow-compensated (FC) and non-FC diffusion gradient waveforms to specifically probe the fraction and velocity of ballistic flow.Methods
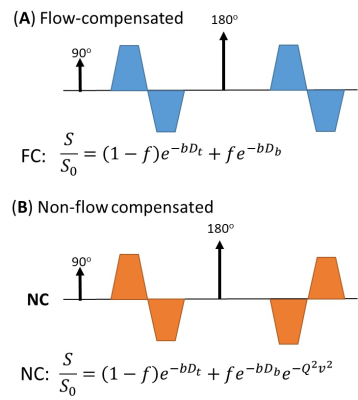
Pulse sequence: House-made FC and NC diffusion sequences were achieved using bipolar diffusion gradients with identical (FC) or mirrored (NC) polarity before and after the 180º refocusing pulse (Figure 1), such that the first-order moment of the FC gradient became zero. We used a gradient duration of 15 ms (effective diffusion time=15 ms).IVIM model: The diffusion signals that take into account the ballistic flow and the tissue water can be represented as: $$$\frac{S}{S_{0}}=(1-f)e^{-bD_{t}}+fe^{-bD_{b}}\cdot e^{-\alpha^{2}v_{b}^{2}}$$$ (according to Ahlgren et al. 4), where Dt and Db are the diffusive coefficients of the water molecules in the tissue and the blood, with Db set to 1.5 µm2/ms (5), and α is the first-order moment of the gradient for flow-sensitization. In the FC sequence, α=0, and therefore, by the joint analysis of FC and NC signals, both f and vb can be resolved. Note that the pseudo-diffusive was not included in the current model, because the diffusion distance of the water molecules in the blood is approximately 9µm at a diffusion time of 15 ms, which is relatively short compared to the vessel segments in the terminal villi 6,7.
Data acquisition: Forty pregnant women (gestational ages 22.7 to 38 weeks) were scanned on a 1.5T MAGNETOM Aera system (Siemens Healthcare, Erlangen, Germany) with an 18-channel body coil. For the IVIM scans, FC and NC gradients were performed at 9 b-values from 10 to 600 s/mm2 in 6 directions, with an FOV of 350×350 mm2, 2.73×2.73 mm2 in-plane resolution, 10 slices of 6 mm slice thickness, and scan time of 2.5 min.
Data analysis: The IVIM parameters were fitted by joint analyses of FC and NC signals using least-square nonlinear curve fitting in MATLAB. Conventional IVIM parameters (f, D, and D*) were fitted separately with the FC and NC data. vb from the joint model, and f∙D* (an analog to velocity 8) from the individual FC or NC data were correlated with the Doppler ultrasound–based pulsatility index (PI) and systolic/diastolic ratio (SD) of the umbilical flow.
Results
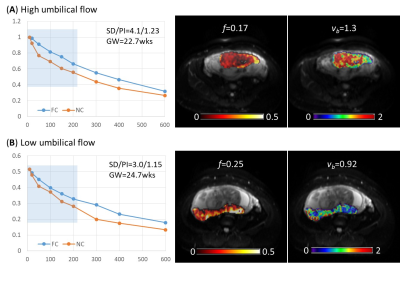
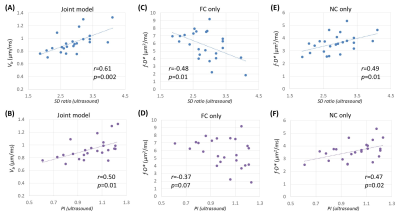
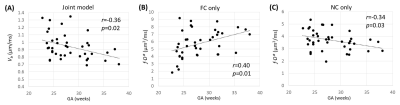
The use of FC and NC sequences led to different IVIM signal decay patterns in the placenta. The discrepancy between the FC- and NC-based signal curves, which resulted from the ballistic flow, varied across the placentas (Figure 2). The placenta with high umbilical flow showed a high flow effect as reflected in the signal curves (evident signal differences between FC and NC data in the low b-value regime) as well as high vb fitted from the joint model; while the one with low umbilical flow showed a low flow effect and low vb (Figure 2). In 25 of the 40 subjects who had ultrasound measurements, we found high correlations between the ultrasound-based flow measurements and vb (Figures 3A-B). Interestingly, the f∙D* values from the FC data demonstrated a negative correlation with SD and PI (Figures 3C-D), and f∙D* values from the NC data demonstrated a positive correlation with SD and PI (Figures 3E-F). In addition, a moderate gestational age dependency was observed for the vb, as well as for the D* and f∙D* values from both FC and NC data (Figure 4).Discussion
Previous studies have reported the use of FC and NC sequences to improve the accuracy of IVIM parameter estimation (f, D, and D*) in the brain, liver, and placenta 4,5,9. These earlier studies typically obtained D from FC measurements and then fitted f and D* with NC data. Here, we used FC and NC data in a joint model and focused on estimating the velocity of ballistic flow, a measurement that is not directly available from conventional IVIM. The fitted results were evaluated by correlation with ultrasound-based umbilical flow. Since the umbilical flow drives the blood flow in the fetal villi, a positive correlation was expected and supported by the experimental data. It should be noted that vb in the current model only reflects the ballistic IVIM compartment (4); whereas the f∙D* is an approximation flow velocity of pseudo-diffusive compartment 8. The fact that f∙D* from the FC data showed an opposite correlation with umbilical flow compared to that of vb indicated that different IVIM compartments may respond differently to the changes in umbilical flow; whereas the interpretation of f∙D* obtained from the NC data using conventional bi-exponential model is more complicated.Conclusion
With the joint use of FC and NC diffusion sequences, we were able to probe the velocity of the ballistic IVIM component in the placenta, which demonstrated a strong correlation with the umbilical flow and moderate correlation with gestational age. This joint approach provides a potential way to noninvasively examine flow velocity in the placental villi for assessment of pregnancy complications.Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Derwig I, Lythgoe DJ, Barker GJ, Poon L, Gowland R, Yeung R, Zelaya F, Nicolaides K. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta 2013;34(10):885-891.
2. Moore RJ, Strachan BK, Tyler DJ, Duncan KR, Baker PN, Worthington BS, Johnson IR, Gowland PA. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo-planar MRI. Placenta 2000;21(7):726-732.
3. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168(2):497-505.
4. Ahlgren A, Knutsson L, Wirestam R, Nilsson M, Stahlberg F, Topgaard D, Lasic S. Quantification of microcirculatory parameters by joint analysis of flow-compensated and non-flow-compensated intravoxel incoherent motion (IVIM) data. NMR in biomedicine 2016;29(5):640-649.
5. Wetscherek A, Stieltjes B, Laun FB. Flow-compensated intravoxel incoherent motion diffusion imaging. Magn Reson Med 2015;74(2):410-419.
6. Plitman Mayo R, Charnock-Jones DS, Burton GJ, Oyen ML. Three-dimensional modeling of human placental terminal villi. Placenta 2016;43:54-60.
7. Erlich A, Pearce P, Mayo RP, Jensen OE, Chernyavsky IL. Physical and geometric determinants of transport in fetoplacental microvascular networks. Science advances 2019;5(4):eaav6326.
8. Le Bihan D, Turner R. The Capillary Network - a Link between Ivim and Classical Perfusion. Magn Reson Med 1992;27(1):171-178.
9. Turk EA, Gagoski B, Stout J, Abulnaga SM, Copeland N, Roberts D, Golland P, Wald L, Adalsteinsson E, Jr WB, Grant PE, Rathi Y. Assessment of placental microcirculation by joint analysis of flow compensated and non-flow compensated intravoxel incoherent motion data. 2019; Montreal, QC, Canada.
Figures