0575
Post-NAC evaluation using ultrafast breast dynamic contrast-enhanced MRI
Maya Honda1, Masako Kataoka1, Mami Iima1, Kanae Kawai Miyake1, Akane Ohashi1, Ayami Ohno Kishimoto1, Rie Ota1, Marcel Dominik Nickel2, Tatsuki Kataoka3, Masakazu Toi4, and Kaori Togashi1
1Department of Diagnostic Imaging and Nuclear Medicine, Graduate School of Medicine, Kyoto University, Kyoto, Japan, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Diagnostic pathology, Graduate School of Medicine, Kyoto University, Kyoto, Japan, 4Department of breast surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
1Department of Diagnostic Imaging and Nuclear Medicine, Graduate School of Medicine, Kyoto University, Kyoto, Japan, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Diagnostic pathology, Graduate School of Medicine, Kyoto University, Kyoto, Japan, 4Department of breast surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Synopsis
The study evaluated the accuracy to predict pathologic complete response (pCR) after neo-adjuvant chemotherapy (NAC) using ultrafast dynamic contrast-enhanced (UF-DCE) MRI. The sensitivity to predict pCR was higher on UF-DCE MRI compared with conventional dynamic contrast-enhanced (DCE) MRI. The difference in image and pathological sizes on UF-DCE MRI was smaller than on conventional DCE MRI. UF-DCE MRI potentially assesses post-NAC status in breast cancer patients accurately in a shorter acquisition time.
Introduction
Pathologic complete response (pCR) after neo-adjuvant chemotherapy (NAC) is an important prognostic factor for breast cancer patients. Pre-surgical prediction of pCR can be used for optimal choice of treatment [1-2]. Moreover, an accurate measurement of residual tumor size after NAC is helpful in determining the appropriate surgical approach. Dynamic contrast-enhanced (DCE) MRI is often used for predicting treatment response, but sometimes fails to predict pCR [3-6]. Currently, there is no consensus on the optimal phase of DCE-MRI for evaluating treatment response. Therefore, in this study, we aimed to evaluate the optimal timing for evaluation including ultrafast DCE (UF-DCE) MRI and conventional DCE-MRI.Methods
Study subjects: Our study population consisted of 28 consecutive female patients who underwent NAC for breast cancer and underwent pre-operative breast DCE MRI with the UF-DCE protocol from April 2016 to October 2019. MR examinations were performed using a 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 16-channel dedicated bilateral breast coil. Gadobutrol (Gadovist, Bayer. Germany) was intravenously infused at a dose of 0.1 ml/kg and at a rate of 2.0 ml/sec, followed by 20 ml of saline at the same rate. The DCE MR protocols were as follows: 1. pre phase; 2. UF-DCE MRI (15 sec before contrast injection-60 sec after contrast injection, 2 sec preparation time followed by 3.7 sec/phase×continuous 20 phases); 3. initial phase of DCE MRI, 60-120 sec after contrast injection (DCE1); 4. high-spatial-resolution contrast-enhanced MRI, 120-300 sec after contrast injection (HR); 5. Delayed phase of DCE MRI, 300-360 sec after contrast injection (DCE2). UF-DCE MRI was acquired with a prototype based on the 3D gradient-echo VIBE sequence using a Compressed Sensing (CS) reconstruction (TR/TE 5.0/2.5 ms, FA 15 degrees, FOV 360 mm×360 mm, matrix 384×269, thickness 2.5 mm, CS acceleration=16.5, temporal resolution 3.7 sec per phase, 20 phases). CS reconstruction was performed with 30 iterations. Images of DCE1, DCE2, HR and the 20th phase of UF-DCE MRI (UF) were used for the image evaluation.Image evaluation: Two independent radiologists evaluated if there was residual enhancing area in each protocol (UF, DCE1, DCE2 and HR) and recorded the maximum length of the enhancing area in the axial plane. When no enhancing area was observed, it was regarded as complete response and recorded as “0”. Images of UF, DCE1, DCE2 and HR were evaluated in this order. Readers were allowed to refer to the pre-NAC MRIs.
Statistical analysis:
1. Diagnostic sensitivity and specificity: The inter-reader agreement in the presence or absence of residual lesion was evaluated using kappa statistics. The sensitivity and specificity of each protocol for predicting pathological complete response (pCR) were calculated. pCR was defined in two ways: 1. No residual tumor (pCR1) and 2. No invasive cancer, in situ carcinoma can be present (pCR2).
2. Comparison in size: The inter-reader agreement for the lesion diameter was evaluated by calculating intraclass correlation coefficients (ICC). The size of the residual lesions on each protocol was compared with that on surgical specimens, and the difference between the image and pathological sizes of each protocol was compared using Wilcoxon signed-rank test with Bonferroni correction. Pathological lesion sizes below 1mm were rounded up to 1mm. The significance level was adjusted by Bonferroni correction.
Results
A total of 28 cases with 29 lesions (one patient had bilateral lesions) was evaluated. Among 29 lesions, 8 lesions achieved pCR1 and 13 lesions achieved pCR2.1. Diagnostic sensitivity and specificity (Figure1): The inter-reader agreement in the presence or absence of residual lesions was almost perfect (kappa values: 0.869–0.922). The sensitivity to predict pCR was the highest on UF, regardless of whether pCR was considered as pCR1 or pCR2. The specificity tended to be inferior on UF except for the result of reader 2 when pCR was considered as pCR2.
2. Comparison in size (Figure 2): The inter-reader agreement in the lesion diameter was excellent (ICC: 0.935–0.984). One lesion was excluded from the size comparison because it remained scattered and the pathological size could not be obtained. Among 28 lesions, the difference in image-based and pathological sizes in UF-DCE MRI was smaller than in conventional DCE MRI and high-resolution contrast-enhanced MRI. The size difference on UF was significantly smaller than that on DCE1, DCE2, and HR, respectively.
Representative cases of pCR1 and pCR2 are shown in Figures 3 and 4.
Discussion
The sensitivity to predict pCR is higher on UF-DCE MRI compared to later phases in conventional breast dynamic imaging, implying lower false negatives using UF-DCE MRI. Residual lesion size on UF-DCE MRI is much closer to pathological lesion size than on the others. Gradually enhancing scar or inflammatory tissue may contribute to overestimation of residual lesion size on conventional DCE MRI or high-resolution contrast-enhanced MRI. Pre-surgical evaluation after NAC using UF-DCE MRI may help minimize the area of resection. Our preliminary study shows the potential of UF-DCE MRI to accurately assess post-NAC status in breast cancer patients in a shorter acquisition time.Conclusion
UF-DCE MRI may help predict pCR or evaluate residual lesion size after NAC in breast cancer patients.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research “Evaluation of Wash in Phase in Breast MRI using Ultrafast Imaging” (Grant Number JP15K09922) and a Grant-in-Aid for Scientific Research on Innovative Areas “Initiative for High-Dimensional Data-Driven Science through Deepening of Sparse Modeling” (MEXT grant numbers 25120002, 25120008).References
- van la Parra RFD, Kuerer HM (2016) Selective elimination of breast cancer surgery in exceptional responders: Historical perspective and current trials. Breast Cancer Res 18:1–8.
- Kuerer HM, Vrancken Peeters MJTFD, Rea DW, et al (2017) Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann Surg Oncol 24:2855–2862.
- Schaefgen B, Mati M, Sinn HP, et al (2016) Can Routine Imaging After Neoadjuvant Chemotherapy in Breast Cancer Predict Pathologic Complete Response? Ann Surg Oncol 23:789–795.
- Kim Y, Sim SH, Park B, et al (2018) Magnetic Resonance Imaging (MRI) Assessment of Residual Breast Cancer After Neoadjuvant Chemotherapy: Relevance to Tumor Subtypes and MRI Interpretation Threshold. Clin Breast Cancer 18:459-467.e1.
- Scheel JR, Kim E, Partridge SC, et al (2018) MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. Am J Roentgenol 210:1376–1385.
- Sener SF, Sargent RE, Lee C, et al (2019) MRI does not predict pathologic complete response after neoadjuvant chemotherapy for breast cancer. J Surg Oncol 903–910.
Figures
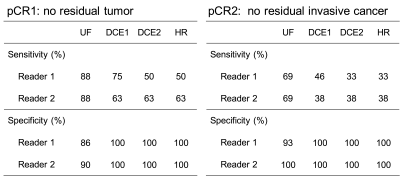
Figure 1: Sensitivity and specificity for predicting pathological complete response (pCR).

Figure 2: The difference in image-based and
pathological sizes on
different phases of breast DCE MRI. The image-based size is the average value of two readers.
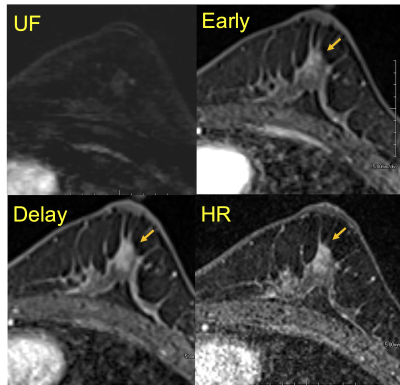
Figure 3: A case of invasive carcinoma of no
special type after neo-adjuvant chemotherapy (NAC). No lesion is detected on UF, but
there seems an enhancing area on the others. Pathological evaluation reveals no residual tumor (pCR1) on surgical specimen.
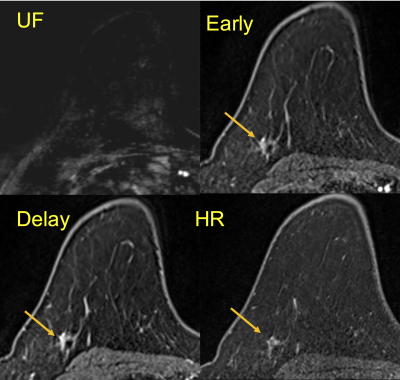
Figure 4:
A
case of invasive carcinoma of no special type after NAC.
No
lesion is detected on UF, but there seems an enhancing area on the others.
All
invasive carcinoma was disappeared; only 7mm
of residual ductal carcinoma in situ was
observed (pCR2) in the surgical specimen.