0564
Optimizing rapid compressed-sensing MPRAGE acquisitions for repeat sampling of brain morphometry within individuals1Center for Brain Science, Harvard University, Cambridge, MA, United States, 2Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Department of Psychology, Harvard University, Cambridge, MA, United States, 4Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 5Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 6LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
Incoherent under-sampling and compressed-sensing reconstructions can reduce the scan time for a 1.0 mm MPRAGE down to as little as 60-90 seconds. Such time-savings permit the acquisition of multiple scans per session, allowing the variation of image metrics and brain morphometrics around their mean values to be quantified. We compared MPRAGE scans accelerated up to eight-fold with a fully-sampled MPRAGE; and using SNR, cortical thickness and gray matter volume, assessed the optimal regularization in the compressed-sensing reconstruction for each acceleration level to best match the values from the fully-sampled scan.
Introduction
Automated MRI-derived morphometrics of human brain volumes and thickness from anatomical scans still require validation. Traditional 1mm isotropic MPRAGE scans take 5-10 minutes to acquire and are therefore highly susceptible to subject motion. Employing rapid scanning techniques have yielded comparable morphometric results to conventional scans1,2,3, and additionally, the ability to acquire multiple images in the same timeframe as one conventional scan. Methods of repeat sampling offer this validation by the ability to quantify variation of the metric around the mean value4,5. Previously, we have investigated the use of incoherent undersampling with compressed-sensing reconstruction to reduce scan times to as little as 72 seconds, with high efficacy6,7,8. But how fast is too fast? Here, we explored optimizing the trade-off between reduced scan time and accuracy of morphometrics, by targeting acceleration factor, regularization factor, and morphometric outputs.Methods
Two participants (mean: 26.9 years, 2 female) completed two imaging sessions within a 7-day period. All T1-weighted images were acquired using a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) and a standard 32-channel head coil. Each session included two conventional MPRAGE (MPR) scans (ADNI-3 parameters but without in-plane acceleration; 9:14 min, TR/TI/TE=2300/900/2.9 ms, matrix 256×240×192, resolution=1mm isotropic). In addition, four sets of four compressed-sensing MPRAGE (csMPR) scans were acquired using a prototype sequence. Imaging parameters were identical to the MPR scan, except for undersampling/acceleration factors of x2, x4, x6 and x8, yielding scan times of 3:43, 1:53, 1:16 and 0:58 min, respectively. Each csMPR scan was retro-reconstructed at the scanner console using 4 different regularization settings to test its effects on the image quality and SNR. The entire process was then repeated within 7 days to control for test-retest variation of acceleration factor and regularization factor on brain morphometrics4,5. This totaled two MPR scans and 16 csMPR scans in each session, 82 images per day, for a total of 164 images per person. Images were analyzed using MRIQC9 for QC metrics and FreeSurfer v6.0.010 after all the scans from each subject were aligned using the FreeSurfer robust registration tool11. SNR, mean cortical thickness and cortical gray matter volume (GMV) are presented here.Results
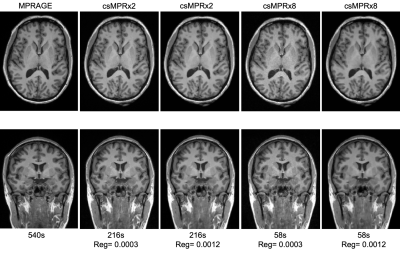
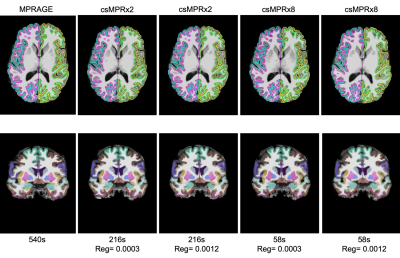
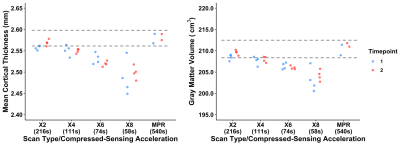
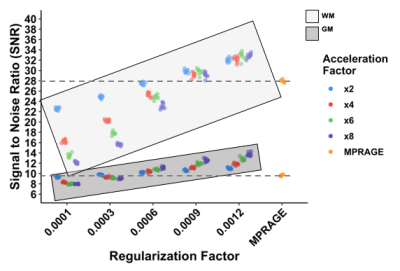
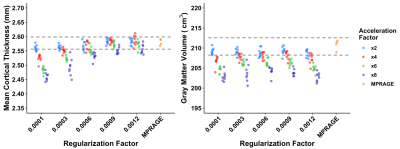
Example MPR and two csMPR scans reconstructed with both low and high regularization are shown in Figure 1. The csMPR x2 scan very closely resembles the MPR regardless of the regularization used; while the x8 scan shows a noticeably lower SNR that can be improved remarkably with increased regularization. The resulting surface delineations and cortical parcellations from each image are shown in Figure 2 – visually the agreement is very high. Mean thickness and GMV as a function of acceleration factor are presented in Figure 3. In this analysis, the default regularization (0.0003) was used in reconstruction. Both metrics show a reduction as a function of acceleration factor, although this relationship may not be linear. At lower levels of acceleration (x2 - x4), brain morphometrics are similar to those obtained from from standard MPR scans. At higher accelerations, a considerable under-estimation is observed. Figure 4 shows the relationship between image SNR, acceleration level and regularization for one subject. While SNR varies greatly between acceleration factors, particularly for white matter, this difference is removed using higher regularization parameters. A regularization value of 0.0009 brings WM SNR for all scans closest to that of the unaccelerated MPR. Perhaps not co-incidentally, mean cortical thickness is most similar to those from MPR scans when a regularization of 0.0009 is used in reconstruction (Fig. 5), while GMV has the smallest spread with regularization of 0.0006. Using an acceleration factor of x8, regardless of regularization parameter, appears to be too under-sampled to show consistent results.Discussion
We have previously demonstrated that csMPR scans can be accelerated up to 6 times faster than a conventional MPR scan with minimal impact on bulk morphometrics8. Here, we have carefully compared the morphometrics obtained from independent repeated single cs-MPR scans with those from a fully-sampled MPR with otherwise identical acquisition parameters. While SNR can be set arbitrarily in images reconstructed using processes like compressed sensing, here we used matched protocols and ensured a lack of other filtering procedures during reconstruction to contrast the output of a csMPR scan with an otherwise identical MPR scan. By tailoring the regularization parameter used in the reconstruction process, we can reduce scatter in repeated measurements of mean cortical thickness and gray matter volume at acceleration values x2 through x6, and have those values closely match those obtained from the MPR scans. In optimizing csMPR for widespread use, a good compromise between noise and image sharpness must be found, and matching SNR and morphometric results to those obtained from a fully-sampled MPR illustrates that csMPR scans can provide image and morphometrics of equivalent quality. With the current implementation, for 1.0 mm isotropic MPRAGE scans, we recommend a regularization 0.0003 for x2 and x4 acceleration, while 0.0006 or 0.0009 appears better for x6 acceleration. Acceleration of x8 or greater appears to be too under-sampled to provide reliable morphometrics. Future work will include an investigation of edge-sharpness to more quantitatively assess the impact of the regularization in the compressed-sensing reconstruction.Acknowledgements
Harvard Center for Brain Science; NIH Shared Instrumentation Grant S10OD020039; NIH Grants P50-MH106435, P41-RR14075, U24-RR021382.References
1. Holmes, A.J. et al., ‘Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures’, Sci. Data 2015;2:150031.
2. Mair, R.W. et al., ‘Quantitative Comparison of Morphometric Data from Multi-Echo MPRAGE with Variable Acceleration and Different Head Coils’, Proceedings ISMRM 2013;21:947.
3. Mair, R.W. et al., ‘Impact of acquisition parameters on cortical thickness and volume derived from Multi-Echo MPRAGE scans’, Proceedings ISMRM 2016;24:1158.
4. Nielsen, J.A. et al., ‘Precision Brain Morphometry: Feasibility and Opportunities of Extreme Rapid Scans’, bioRxiv 2019;530436.
5. Hanford, L.C. et al., ‘Proof-of-Concept Longitudinal Tracking of Brain Change Within Individual Patients Using Repeated Acquisitions of Rapid Structural Scans’, Proceedings Society for Neuroscience 2019;427.19.
6. Lustig M. et al., ‘Sparse MRI: The application of compressed sensing for rapid MR imaging’ Magn. Reson. Med. 2007;58:1182-1195.
7. Mussard E. et al., ‘Accelerated MP2RAGE Imaging Using Sparse Iterative Reconstruction’, Proceedings ISMRM 2016;24:4216.
8. Mair, R.W. et al., ‘Towards 1 min brain morphometery – evaluating compressed-sensing MPRAGE, Proceedings ISMRM 2019;27:2978.
9. Esteban O. et al., ‘MRIQC: Advancing the Automatic Prediction of Image Quality in MRI from Unseen Sites’, PLOS ONE 2017;12:e0184661.
10. Fischl, B., ‘Measuring the thickness of the human cerebral cortex from magnetic resonance images’, Proc. Natl. Acad. Sci. USA, 2000;97:11050-11055.
11. Reuter, M., ‘Highly Accurate Inverse Consistent Registration: A Robust Approach’, NeuroImage, 2010;53;1181-1196.
Figures