0559
Accelerated 3D multiparametric MRI in glioma patients - Initial clinical experience1Informatics, Technical University of Munich, Munich, Germany, 2GE Healthcare, Munich, Germany, 3Radiology & Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands, 4Fondazione Imago7, Pisa, Italy, 5IRCCS Fondazione Stella Maris, Pisa, Italy, 6Physics, Technical University of Munich, Munich, Germany
Synopsis
In brain tumor diagnosis, fully quantitative, multiparametric MRI offers great opportunities as it allows for comprehensive tissue and hence tumor characterization which is essential for treatment planning and monitoring the treatment response. With its highly accelerated acquisition, advanced rapid MR mapping techniques facilitate multiparametric imaging in clinically acceptable scan times, providing quantitative, reproducible and accurate diagnostic information that is less affected by system and interpretation biases. In this work, we present initial clinical results and demonstrate the feasibility of a novel 3D multiparametric quantitative transient-state imaging (QTI) acquisition scheme in glioma patients.
Introduction
Due to its superior soft-tissue contrast, MRI is the method of choice for tissue characterization in brain tumor diagnosis. It also guides treatment planning and is required to monitor treatment response. Here quantitative MRI offers great opportunities as it provides comprehensive diagnostic information. Owing to long acquisition times of conventional quantitative MR techniques, routine imaging protocols for brain tumors mainly present qualitative information.1 The limitations arising from qualitative imaging and interpretation also apply to the process of identifying and describing tumor sub-structures of interest. That is, the rich patho-physiological information of advanced clinical imaging sequences is underutilized, as analyzing the complex multi-parametric, multimodal and even multi-temporal image data sets remains a major challenge. Aiming for quantitative imaging biomarkers, advanced rapid multiparametric mapping techniques2–5 have recently been demonstrated to facilitate multiparametric imaging in clinical acceptable scan times, providing quantitative, reproducible and accurate diagnostic information which is less affected by system and interpretation biases.In this work, we present initial clinical results of a novel 3D multiparametric quantitative transient-state imaging (QTI) acquisition scheme in glioma patients (grade II-IV).
Methods
As part of an IRB-approved study, data from three glioma patients were acquired on a 3T MR750 system (GE Healthcare, Milwaukee, WI) after obtaining written informed consent. The MR protocol included conventional, qualitative and quantitative sequences. In addition to the clinical sequences, the patients were scanned with the proposed 3D QTI acquisition which is based on the following schedule: after an initial adiabatic inversion (TI=18ms), flip angles (0.8°≤FA≤70°) are applied in a ramp-up/down pattern with TR/TE=7.8ms/1.8ms and 880 repetitions using a varying-density spiral readout (22.5x22.5x22.5cm3 FOV, 1.25x1.25x1.25mm3 isotropic resolution) with in-plane and spherical rotations.6 In-plane rotations were incremented with the golden angle from one repetition to the next. Spherical rotations were applied to acquire data in all three spatial dimensions with fully sampled in-plane discs. 3D QTI data was reconstructed using k-space weighted view-sharing7 and zero-filling, respectively, followed by dimensionality reduction via SVD subspace projection, gridding onto a Cartesian grid using gpuNUFFT8, 3D FFT and subsequent coil sensitivity correction.We obtained quantitative maps of T1, T2 and PD by matching the reconstructed subspace images to a dictionary which was computed for T1=[100:20:4000]ms and T2=[20:4:600]ms using the Extended Phase Graphs formalism9.
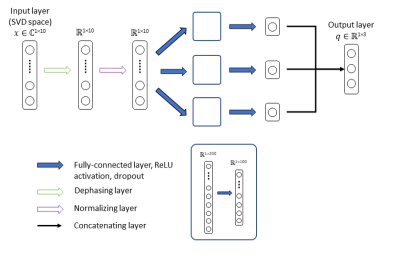
As an alternative to exhaustive dictionary matching, we trained a neural network to infer T1, T2 and PD estimates. As illustrated in Figure 1, the proposed model receives the first 10 singular components of the SVD compressed QTI signal x as input and outputs the underlying tissue parameters q, i.e. T1, T2 and a PD-related scaling factor, with PD=||x||2/q3. We trained the model on 70% of the samples in the simulated dataset with added Gaussian noise, using ReLU activation, L1 loss and ADAM optimization (learning rate=1e-4, dropout rate=0.8, 1200 epochs).
Results
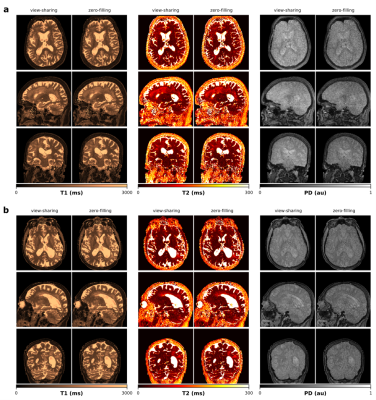
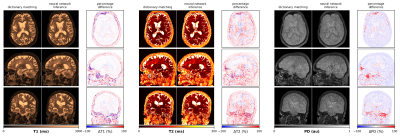
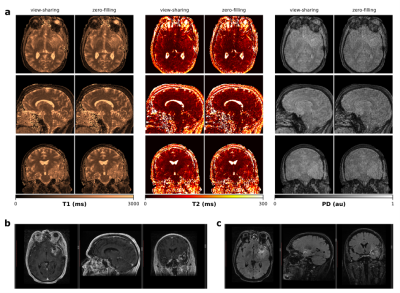
Isotropic 3D maps of T1, T2 and PD obtained with the view-sharing and zero-filling reconstruction, respectively, and subsequent dictionary matching are shown in Figure 2. Figure 3 shows that parametric maps obtained via fast neural network inference (computation time of less than 1min) are largely consistent with the dictionary matching results (computation time of 16min). That is, combining GPU gridding, zero-filling and neural network inference in the reconstruction pipeline, we achieved a total reconstruction time of less than 7min. As seen from Figure 4, patient movement can affect the estimation of tissue parameters. In case of head motion, 3D QTI achieves an image quality comparable to state-of-the-art FSPGR and CUBE FLAIR sequences.Discussion
Initial experience with 3D QTI in glioma patients demonstrated fully quantitative, multiparametric MR mapping with high isotropic resolution and an acquisition time that make it feasible for use under tight clinical time-constraints. Combining the 3D QTI framework with a neural network for T1, T2 and PD inference proves to be a fast and memory-efficient alternative to a conventional dictionary matching approach. Once trained, the neural network offers fast parameter quantification that is not restricted by the size or granularity of the dictionary. 3D QTI demonstrated to reliably identify tissue and hence tumor heterogeneity that is captured in T1, T2 and PD parameters. As such, it offers comprehensive tissue assessment of tumor sub-structures with the potential to improve disease characterization in brain tumor patients. This is essential to find the optimal treatment strategy and to monitor treatment response along the course of disease.With its fast acquisition, 3D QTI achieves a motion robustness comparable to qualitative, state-of-the-art protocols which is particularly advantageous for severely diseased patients with difficulties to lie still during lengthy scanning sessions. Initial experience, however, also showed that severe patient motion degrades the image quality of estimated parameter maps. We are optimistic that combining the 3D QTI framework with a motion correction algorithm10 can further improve its robustness and is hence subject of future work.
Conclusion
This work demonstrates 3D multiparametric quantitative transient-state imaging (QTI) in glioma patients. The framework offers quantitative MRI with acquisition and reconstruction times that make it suitable for application in clinical practice. Its potential to provide fast and quantitative tissue characterization with high isotropic resolution may be particularly beneficial for monitoring treatment response in brain tumor patients.Acknowledgements
Carolin M Pirkl is supported by Deutsche Forschungsgemeinschaft (DFG) through TUM International Graduate School of Science and Engineering (IGSSE), GSC 81.References
1. Thust SC, Heiland S, Falini A, et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018;28(8):3306–3317.
2. Gómez PA, Molina-Romero M, Buonincontri G, et al. Designing contrasts for rapid, simultaneous parameter quantification and flow visualization with quantitative transient-state imaging. Sci. Rep. 2019;9(1):8468.
3. Cao X, Ye H, Liao C, et al. Fast 3D brain MR fingerprinting based on multi-axis spiral projection trajectory. Magn. Reson. Med. 2019;82(1):289–301.
4. Sbrizzi A, van der Heide O, Cloos M, et al. Fast quantitative MRI as a nonlinear tomography problem. Magn. Reson. Imaging. 2018;46:56–63.
5. Warntjes JBM, Leinhard OD, West J, et al. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn. Reson. Med. 2008;60(2):320–329.
6. Cao X, Ye H, Liao C, et al. Fast 3D brain MR fingerprinting based on multi-axis spiral projection trajectory. Magn. Reson. Med. 2019;82(1):289–301.
7. Bounincontri G, Biagi Laura, Gómez PA, et al. Spiral keyhole imaging for MR fingerprinting. In: Proc Intl Soc Mag Reson Med. Honolulu, HI, USA; 2017.
8. Knoll F, Schwarzl A, Diwoky C, et al. gpuNUFFT - An Open-Source GPU Library for 3D Gridding with Direct Matlab Interface. In: Proc Intl Soc Mag Reson Med. Milan, Italy; 2014.
9. Weigel M. Extended phase graphs: dephasing, RF pulses, and echoes - pure and simple. J. Magn. Reson. Imaging JMRI. 2015;41(2):266–295.
10. Kurzawski JW, Cencini M, Gómez PA, et al. Three-dimensional motion correction in Magnetic Resonance Fingerprinting (MRF). In: Proc Intl Soc Mag Reson Med. Montréal, QC, Canada; 2019.
Figures